Long-term trends in total administered radiation dose from brain [18F]FDG-PET in children with drug-resistant epilepsy
- PMID: 39352423
- PMCID: PMC11732939
- DOI: 10.1007/s00259-024-06902-8
Long-term trends in total administered radiation dose from brain [18F]FDG-PET in children with drug-resistant epilepsy
Abstract
Purpose: To assess the trends in administered 2-[18F]fluoro-2-deoxy-D-glucose ([18F]FDG) doses, computed tomography (CT) radiation doses, and image quality over the last 15 years in children with drug-resistant epilepsy (DRE) undergoing hybrid positron emission tomography (PET) brain scans.
Methods: We retrospectively analyzed data from children with DRE who had [18F]FDG-PET/CT or magnetic resonance scans for presurgical evaluation between 2005 and 2021. We evaluated changes in injected [18F]FDG doses, administered activity per body weight, CT dose index volume (CTDIvol), and dose length product (DLP). PET image quality was assessed visually by four trained raters. Conversely, CT image quality was measured using region-of-interest analysis, normalized by signal-to-noise (SNR) and contrast-to-noise ratio (CNR).
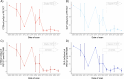
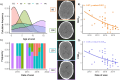
Results: We included 55 children (30 male, mean age: 9 ± 6 years) who underwent 61 [18F]FDG-PET scans (71% as PET/CT). Annually, the injected [18F]FDG dose decreased by ~ 1% (95% CI: 0.92%-0.98%, p < 0.001), with no significant changes in administered activity per body weight (p = 0.51). CTDIvol and DLP decreased annually by 16% (95% CI: 9%-23%) and 15% (95% CI: 8%-21%, both p < 0.001), respectively. PET image quality improved by 9% year-over-year (95% CI: 6%-13%, p < 0.001), while CT-associated SNR and CNR decreased annually by 7% (95% CI: 3%-11%, p = 0.001) and 6% (95% CI: 2%-10%, p = 0.008), respectively.
Conclusion: Our findings indicate stability in [18F]FDG administered activity per body weight alongside improvements in PET image quality. Conversely, CT-associated radiation doses reduced. These results reaffirm [18F]FDG-PET as an increasingly safer and higher-resolution auxiliary imaging modality for children with DRE. These improvements, driven by technological advancements, may enhance the diagnostic precision and patient outcomes in pediatric epilepsy surgery.
Keywords: Drug-resistant epilepsy; Image quality; Pediatric; Radiation dose; [18F]FDG-PET.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Informed consent and ethics approval: The present study was approved by the local ethics committee (Number: 2020–03067) and was conducted in compliance with ICH-GCP rules and the Declaration of Helsinki. Written informed consent was waived for patients whose scan was acquired before January 2016. After January 2016, only patients with documented consent to the use of their medical data for research were included. Consent to publish: The datasets analyzed in the current study relies on brain scans, which hardly contain identifying characteristics, representing one of the exceptions to obtaining consent to publish. Competing interest: The University Hospital Zurich holds a research agreement with GE Healthcare (unrelated to the current study). PD Dr. Martin W. Hüllner is a recipient of research grants by GE Healthcare. Apart from that, the authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Figures

References
-
- Aaberg KM, Gunnes N, Bakken IJ, Lund Soraas C, Berntsen A, Magnus P, et al. Incidence and Prevalence of Childhood Epilepsy: A Nationwide Cohort Study. Pediatrics. 2017;139. 10.1542/peds.2016-3908. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

