Incontinentia pigmenti underlies thymic dysplasia, autoantibodies to type I IFNs, and viral diseases
- PMID: 39352576
- PMCID: PMC11448874
- DOI: 10.1084/jem.20231152
Incontinentia pigmenti underlies thymic dysplasia, autoantibodies to type I IFNs, and viral diseases
Abstract
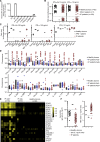
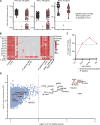
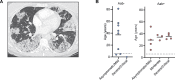
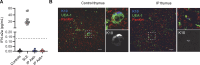
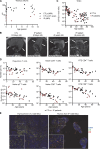
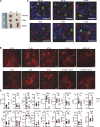
Human inborn errors of thymic T cell tolerance underlie the production of autoantibodies (auto-Abs) neutralizing type I IFNs, which predispose to severe viral diseases. We analyze 131 female patients with X-linked dominant incontinentia pigmenti (IP), heterozygous for loss-of-function (LOF) NEMO variants, from 99 kindreds in 10 countries. Forty-seven of these patients (36%) have auto-Abs neutralizing IFN-α and/or IFN-ω, a proportion 23 times higher than that for age-matched female controls. This proportion remains stable from the age of 6 years onward. On imaging, female patients with IP have a small, abnormally structured thymus. Auto-Abs against type I IFNs confer a predisposition to life-threatening viral diseases. By contrast, patients with IP lacking auto-Abs against type I IFNs are at no particular risk of viral disease. These results suggest that IP accelerates thymic involution, thereby underlying the production of auto-Abs neutralizing type I IFNs in at least a third of female patients with IP, predisposing them to life-threatening viral diseases.
© 2024 Rosain et al.
Conflict of interest statement
Disclosures: A.V. Parent reported personal fees from Thymmune Therapeutics outside the submitted work; in addition, A.V. Parent had a patent to generate thymic epithelial progenitor cells in vitro licensed (Thymmune Therapeutics). J.D. Milner reported personal fees from Blueprint Pharmaceuticals and grants from Pharming outside the submitted work. M.S. Anderson reported other from Merck, Inc. and Medtronic, Inc. outside the submitted work. No other disclosures were reported.
Figures




References
-
- Abers, M.S., Rosen L.B., Delmonte O.M., Shaw E., Bastard P., Imberti L., Quaresima V., Biondi A., Bonfanti P., Castagnoli R., et al. 2021. Neutralizing type-I interferon autoantibodies are associated with delayed viral clearance and intensive care unit admission in patients with COVID-19. Immunol. Cell Biol. 99:917–921. 10.1111/imcb.12495 - DOI - PMC - PubMed
-
- Acosta-Ampudia, Y., Monsalve D.M., Rojas M., Rodríguez Y., Gallo J.E., Salazar-Uribe J.C., Santander M.J., Cala M.P., Zapata W., Zapata M.I., et al. 2021. COVID-19 convalescent plasma composition and immunological effects in severe patients. J. Autoimmun. 118:102598. 10.1016/j.jaut.2021.102598 - DOI - PMC - PubMed
-
- Akbil, B., Meyer T., Stubbemann P., Thibeault C., Staudacher O., Niemeyer D., Jansen J., Mühlemann B., Doehn J., Tabeling C., et al. 2022. Early and rapid identification of COVID-19 patients with neutralizing type I interferon auto-antibodies. J. Clin. Immunol. 42:1111–1129. 10.1007/s10875-022-01252-2 - DOI - PMC - PubMed
-
- Alotaibi, F., Alharbi N.K., Rosen L.B., Asiri A.Y., Assiri A.M., Balkhy H.H., Al Jeraisy M., Mandourah Y., AlJohani S., Al Harbi S., et al. 2023. Type I interferon autoantibodies in hospitalized patients with Middle East respiratory syndrome and association with outcomes and treatment effect of interferon beta-1b in MIRACLE clinical trial. Influenza Other Respir. Viruses. 17:e13116. 10.1111/irv.13116 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
- EQU201903007798/French Foundation for Medical Research
- Rockefeller University
- National Institute of Allergy and Infectious Diseases
- JP22fk0108514/Japan Agency for Medical Research and Development
- ANR-17-EURE-0003/University Grenoble Alpes
- Sidra Medicine
- Chan-Zuckerberg Biohub
- Imagine Institute
- R01 AI163029/AI/NIAID NIH HHS/United States
- 824110/European Union's Horizon 2020 research and innovation program
- K01 AG072789/AG/NIA NIH HHS/United States
- PE00000007/Next Generation EU-MUR PNRR
- Filière Santé Maladies Rares Dermatologiques
- 2141/Göran Gustafsson Foundation
- 101057100/HORIZON-HLTH-2021-DISEASE-04
- National Center for Advancing Sciences
- UL1 TR001866/TR/NCATS NIH HHS/United States
- 22H03041/Japan Society for the Promotion of Science
- R01AI163029/NH/NIH HHS/United States
- Grandir - Fonds de solidarité pour l'enfance
- 2021-03118/Swedish Research Council
- UL1TR001866/RR/NCRR NIH HHS/United States
- Institut National de la Santé et de la Recherche Médicale
- French Ministry of Higher Education, Research, and Innovation
- HHMI/Howard Hughes Medical Institute/United States
- Square Foundation
- NPRP9-251-3-045/Qatar National Research Fund
- Association Incontinentia Pigmenti France
- SCOR Corporate Foundation for Science
- St. Giles Foundation
- Bettencourt-Schueller Foundation
- General Atlantic Foundation
- ANR-10-IAHU-01/French National Research Agency
- Paris Cité University
LinkOut - more resources
Full Text Sources
Miscellaneous

