Aligned Ion Conduction Pathway of Polyrotaxane-Based Electrolyte with Dispersed Hydrophobic Chains for Solid-State Lithium-Oxygen Batteries
- PMID: 39352589
- PMCID: PMC11445217
- DOI: 10.1007/s40820-024-01535-w
Aligned Ion Conduction Pathway of Polyrotaxane-Based Electrolyte with Dispersed Hydrophobic Chains for Solid-State Lithium-Oxygen Batteries
Abstract
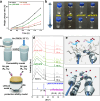
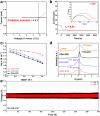
A critical challenge hindering the practical application of lithium-oxygen batteries (LOBs) is the inevitable problems associated with liquid electrolytes, such as evaporation and safety problems. Our study addresses these problems by proposing a modified polyrotaxane (mPR)-based solid polymer electrolyte (SPE) design that simultaneously mitigates solvent-related problems and improves conductivity. mPR-SPE exhibits high ion conductivity (2.8 × 10-3 S cm-1 at 25 °C) through aligned ion conduction pathways and provides electrode protection ability through hydrophobic chain dispersion. Integrating this mPR-SPE into solid-state LOBs resulted in stable potentials over 300 cycles. In situ Raman spectroscopy reveals the presence of an LiO2 intermediate alongside Li2O2 during oxygen reactions. Ex situ X-ray diffraction confirm the ability of the SPE to hinder the permeation of oxygen and moisture, as demonstrated by the air permeability tests. The present study suggests that maintaining a low residual solvent while achieving high ionic conductivity is crucial for restricting the sub-reactions of solid-state LOBs.
Keywords: Hydrophobic chain; Lithium-oxygen batteries; Polyrotaxane ion conductivity; Solid polymer electrolyte.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no interest conflict. They have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




References
-
- C. Shu, J. Long, S.-X. Dou, J. Wang, Component-interaction reinforced quasi-solid electrolyte with multifunctionality for flexible Li–O2 battery with superior safety under extreme conditions. Small 15, e1804701 (2019). 10.1002/smll.201804701 - PubMed
-
- P. Tan, M. Liu, Z. Shao, M. Ni, Recent advances in perovskite oxides as electrode materials for nonaqueous lithium–oxygen batteries. Adv. Energy Mater. 7, 1602674 (2017). 10.1002/aenm.201602674
-
- T. Liu, J.P. Vivek, E.W. Zhao, J. Lei, N. Garcia-Araez et al., Current challenges and routes forward for nonaqueous lithium-air batteries. Chem. Rev. 120, 6558–6625 (2020). 10.1021/acs.chemrev.9b00545 - PubMed
-
- C.H. Kim, M.-C. Sung, B. Hwang, D.-W. Kim, Cu3P nanoarrays derived from 7, 7, 8, 8-tetracyanoquinodimethane for high-rate electrocatalytic oxygen reactions of lithium-oxygen batteries. Int. J. Energy Res. 2024, 1756429 (2024). 10.1155/2024/1756429
LinkOut - more resources
Full Text Sources
