Dual targeting macrophages and microglia is a therapeutic vulnerability in models of PTEN-deficient glioblastoma
- PMID: 39352749
- PMCID: PMC11563674
- DOI: 10.1172/JCI178628
Dual targeting macrophages and microglia is a therapeutic vulnerability in models of PTEN-deficient glioblastoma
Abstract
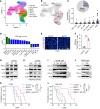
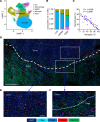
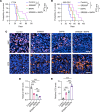
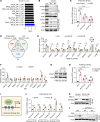
Tumor-associated macrophages and microglia (TAMs) are critical for tumor progression and therapy resistance in glioblastoma (GBM), a type of incurable brain cancer. We previously identified lysyl oxidase (LOX) and olfactomedin like-3 (OLFML3) as essential macrophage and microglia chemokines, respectively, in GBM. Here, single-cell transcriptomics and multiplex sequential immunofluorescence followed by functional studies demonstrate that macrophages negatively correlate with microglia in the GBM tumor microenvironment. LOX inhibition in PTEN-deficient GBM cells upregulates OLFML3 expression via the NF-κB-PATZ1 signaling pathway, inducing a compensatory increase of microglia infiltration. Dual targeting macrophages and microglia via inhibition of LOX and the CLOCK-OLFML3 axis generates potent antitumor effects and offers a complete tumor regression in more than 60% of animals when combined with anti-PD1 therapy in PTEN-deficient GBM mouse models. Thus, our findings provide a translational triple therapeutic strategy for this lethal disease.
Keywords: Brain cancer; Cancer immunotherapy; Macrophages; Oncology.
Conflict of interest statement
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

