Neutrophil-specific Shp1 loss results in lethal pulmonary hemorrhage in mouse models of acute lung injury
- PMID: 39352872
- PMCID: PMC11645157
- DOI: 10.1172/JCI183161
Neutrophil-specific Shp1 loss results in lethal pulmonary hemorrhage in mouse models of acute lung injury
Abstract
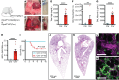
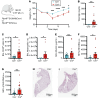
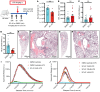
Acute respiratory distress syndrome (ARDS) is associated with significant morbidity and mortality, and neutrophils are critical to its pathogenesis. Neutrophil activation is closely regulated by inhibitory tyrosine phosphatases including Src homology region 2 domain-containing phosphatase-1 (Shp1). Here, we report that loss of neutrophil Shp1 in mice produced hyperinflammation and lethal pulmonary hemorrhage in sterile inflammation and pathogen-induced models of acute lung injury (ALI) through a Syk kinase-dependent mechanism. We observed large intravascular neutrophil clusters, perivascular inflammation, and excessive neutrophil extracellular traps in neutrophil-specific Shp1-KO mice, suggesting an underlying mechanism for the observed pulmonary hemorrhage. Targeted immunomodulation through the administration of a Shp1 activator (SC43) reduced agonist-induced reactive oxygen species in vitro and ameliorated ALI-induced alveolar neutrophilia and NETs in vivo. We propose that the pharmacologic activation of Shp1 has the potential to fine tune neutrophil hyperinflammation that is central to the pathogenesis of ARDS.
Keywords: Bacterial infections; Immunology; Neutrophils; Phosphoprotein phosphatases; Pulmonology.
Conflict of interest statement
Figures



Comment in
- Balancing immune response: SHP1 controls neutrophil activation in inflamed lungs
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

