Amyloid-β oligomers trigger sex-dependent inhibition of GIRK channel activity in hippocampal neurons in mice
- PMID: 39353038
- PMCID: PMC11600338
- DOI: 10.1126/scisignal.ado4132
Amyloid-β oligomers trigger sex-dependent inhibition of GIRK channel activity in hippocampal neurons in mice
Abstract
Alzheimer's disease (AD) is a progressive neurodegenerative disease characterized by amyloid plaques and cognitive decline, the latter of which is thought to be driven by soluble oligomeric amyloid-β (oAβ). The dysregulation of G protein-gated inwardly rectifying K+ (GIRK; also known as Kir3) channels has been implicated in rodent models of AD. Here, seeking mechanistic insights, we uncovered a sex-dependent facet of GIRK-dependent signaling in AD-related amyloid pathophysiology. Synthetic oAβ1-42 suppressed GIRK-dependent signaling in hippocampal neurons from male mice, but not from female mice. This effect required cellular prion protein, the receptor mGluR5, and production of arachidonic acid by the phospholipase PLA2. Although oAβ suppressed GIRK channel activity only in male hippocampal neurons, intrahippocampal infusion of oAβ or genetic suppression of GIRK channel activity in hippocampal pyramidal neurons impaired performance on a memory test in both male and female mice. Moreover, genetic enhancement of GIRK channel activity in hippocampal pyramidal neurons blocked oAβ-induced cognitive impairment in both male and female mice. In APP/PS1 AD model mice, GIRK-dependent signaling was diminished in hippocampal CA1 pyramidal neurons from only male mice before cognitive deficit was detected. However, enhancing GIRK channel activity rescued cognitive deficits in older APP/PS1 mice of both sexes. Thus, whereas diminished GIRK channel activity contributes to cognitive deficits in male mice with increased oAβ burden, enhancing its activity may have therapeutic potential for both sexes.
Conflict of interest statement
Figures
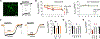
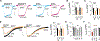




References
-
- Busche MA, Konnerth A, Neuronal hyperactivity--A key defect in Alzheimer’s disease? Bioessays 37, 624–632 (2015). - PubMed
-
- Morra JH, Tu Z, Apostolova LG, Green AE, Avedissian C, Madsen SK, Parikshak N, Hua X, Toga AW, Jack CR Jr., Schuff N, Weiner MW, Thompson PM, Alzheimer’s Disease Neuroimaging I, Automated 3D mapping of hippocampal atrophy and its clinical correlates in 400 subjects with Alzheimer’s disease, mild cognitive impairment, and elderly controls. Hum. Brain. Mapp. 30, 2766–2788 (2009). - PMC - PubMed
-
- Huijbers W, Mormino EC, Schultz AP, Wigman S, Ward AM, Larvie M, Amariglio RE, Marshall GA, Rentz DM, Johnson KA, Sperling RA, Amyloid-beta deposition in mild cognitive impairment is associated with increased hippocampal activity, atrophy and clinical progression. Brain 138, 1023–1035 (2015). - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

