Eculizumab Use in Neuromyelitis Optica Spectrum Disorders: Routine Clinical Care Data From a European Cohort
- PMID: 39353149
- PMCID: PMC11449397
- DOI: 10.1212/WNL.0000000000209888
Eculizumab Use in Neuromyelitis Optica Spectrum Disorders: Routine Clinical Care Data From a European Cohort
Abstract
Background and objectives: Attack prevention is crucial in managing neuromyelitis optica spectrum disorders (NMOSDs). Eculizumab (ECU), an inhibitor of the terminal complement cascade, was highly effective in preventing attacks in a phase III trial of aquaporin-4 (AQP4)-IgG seropositive(+) NMOSDs. In this article, we evaluated effectiveness and safety of ECU in routine clinical care.
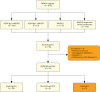
Methods: We retrospectively evaluated patients with AQP4-IgG+ NMOSD treated with ECU between December 2014 and April 2022 at 20 German and 1 Austrian university center(s) of the Neuromyelitis Optica Study Group (NEMOS) by chart review. Primary outcomes were effectiveness (assessed using annualized attack rate [AAR], MRI activity, and disability changes [Expanded Disability Status Scale {EDSS}]) and safety (including adverse events, mortality, and attacks after meningococcal vaccinations), analyzed by descriptive statistics.
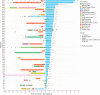
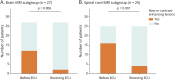
Results: Fifty-two patients (87% female, age 55.0 ± 16.3 years) received ECU for 16.2 (interquartile range [IQR] 9.6 - 21.7) months. Forty-five patients (87%) received meningococcal vaccination before starting ECU, 9 with concomitant oral prednisone and 36 without. Seven of the latter (19%) experienced attacks shortly after vaccination (median: 9 days, IQR 6-10 days). No postvaccinal attack occurred in the 9 patients vaccinated while on oral prednisone before starting ECU and in 25 (re-)vaccinated while on ECU. During ECU therapy, 88% of patients were attack-free. The median AAR decreased from 1.0 (range 0-4) in the 2 years preceding ECU to 0 (range 0-0.8; p < 0.001). The EDSS score from start to the last follow-up was stable (median 6.0), and the proportion of patients with new T2-enhancing or gadolinium-enhancing MRI lesions in the brain and spinal cord decreased. Seven patients (13%) experienced serious infections. Five patients (10%; median age 53.7 years) died on ECU treatment (1 from myocardial infarction, 1 from ileus with secondary sepsis, and 3 from systemic infection, including 1 meningococcal sepsis), 4 were older than 60 years and severely disabled at ECU treatment start (EDSS score ≥ 7). The overall discontinuation rate was 19%.
Discussion: Eculizumab proved to be effective in preventing NMOSD attacks. An increased risk of attacks after meningococcal vaccination before ECU start and potentially fatal systemic infections during ECU-particularly in patients with comorbidities-must be considered. Further research is necessary to explore optimal timing for meningococcal vaccinations.
Classification of evidence: This study provides Class IV evidence that eculizumab reduces annualized attack rates and new MRI lesions in AQP4-IgG+ patients with NMOSD.
Conflict of interest statement
M. Ringelstein received speaker honoraria from Novartis, Bayer Vital GmbH, Roche, Alexion, Horizon, and Ipsen and travel reimbursement from Bayer Schering, Biogen Idec, Merz, Genzyme, Teva, Roche, Horizon, Alexion, and Merck, none related to this study. S. Asseyer received speaker honoraria from Alexion, Bayer GmbH, and Roche, not related to this study. G. Lindenblatt received travel reimbursement from Bayer Health Care, not related to this study. K. Fischer reports no disclosures relevant to the manuscript. R. Pul received speaker’s and board honoraria from Alexion, Bayer Healthcare, Biogen, Celgene, Janssen Cilag, Merck Serono, Mylan/Viatris, Novartis, Roche, Sanofi Genzyme/Aventis, Stada, and Teva, and received research grants from Herz Burgdorf, Novartis, and Merck, none related to the content of this study. J. Skuljec, L. Lohmann, and K. Giglhuber report no disclosures relevant to the manuscript. V. Häußler reports funding from NEMOS e.V. independent of this project. M. Karenfort received board honoraria from Novartis, not related to the content of this manuscript. K. Hellwig received speaker’s, board honoraria and research support from Biogen Idec, Genzyme, Merck Serono, Novartis, Roche, Hexal, and Teva; and her institution received grant support from Hexal, Biogen Idec, Sanofi-Genzyme, Merck Serono, Novartis, Roche, and Teva. F. Paul serves as an academic editor for
Figures

References
-
- Jarius S, Aktas O, Ayzenberg I, et al. . Update on the diagnosis and treatment of neuromyelits optica spectrum disorders (NMOSD) – revised recommendations of the neuromyelitis optica study group (NEMOS). part I: diagnosis and differential diagnosis. J Neurol. 2023;270(7):3341-3368. doi:10.1007/s00415-023-11634-0 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
