Efficacy and Safety of Roxadustat for Anemia in Patients Receiving Chemotherapy for Nonmyeloid Malignancies: A Randomized, Open-Label, Active-Controlled Phase III Study
- PMID: 39353163
- PMCID: PMC11708981
- DOI: 10.1200/JCO.23.02742
Efficacy and Safety of Roxadustat for Anemia in Patients Receiving Chemotherapy for Nonmyeloid Malignancies: A Randomized, Open-Label, Active-Controlled Phase III Study
Abstract
Purpose: We evaluated the efficacy and safety of roxadustat, a first-in-class hypoxia-inducible factor prolyl hydroxylase inhibitor, for chemotherapy-induced anemia (CIA) in patients with nonmyeloid malignancies receiving multicycle treatments of chemotherapy.
Patients and methods: In this open-label, noninferiority phase III study conducted at 44 sites in China, 159 participants age ≥18 years with CIA nonmyeloid malignancy and CIA were randomly assigned (1:1) to oral roxadustat or subcutaneous recombinant human erythropoietin-α (rHuEPO-α) three times a week for 12 weeks. Roxadustat starting dosages were 100, 120, and 150 mg three times a week for participants weighing 40-<50, 50-60, and >60 kg, respectively. rHuEPO-α starting dosage for all participants was 150 IU/kg three times a week. Both roxadustat and rHuEPO-α dosages could be modified. The primary end point was least-squares mean (LSM) change in hemoglobin (Hb) concentration from baseline to the concentration averaged over weeks 9-13.
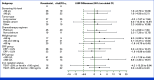
Results: Of the 159 participants randomly assigned, 140 were included in the per-protocol set (roxadustat, n = 78; rHuEPO-α, n = 62). The LSM (95% two-sided CI) change from baseline to weeks 9-13 in Hb concentration was 17.1 (13.58 to 20.71) g/L with roxadustat and 15.4 (11.34 to 19.50) g/L with rHuEPO-α (mean difference [95% CI], 1.7 [-3.39 to 6.84]). The lower bound of the one-sided 97.5% CI for the treatment difference (‒3.4 g/L) was greater than the predefined noninferiority margin of ‒6.6 g/L, establishing noninferiority. Noninferiority was supported by five of six key secondary end points. Rates of adverse events were generally comparable between treatments and consistent with previous findings.
Conclusion: Roxadustat was noninferior to rHuEPO-α in treating CIA in participants with nonmyeloid malignancies receiving multicycle treatments of myelosuppressive chemotherapy. The oral formulation of roxadustat may potentially increase compliance.
Trial registration: ClinicalTrials.gov NCT05301517.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO’s conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
No other potential conflicts of interest were reported.
Figures


References
-
- Groopman JE, Itri LM: Chemotherapy-induced anemia in adults: Incidence and treatment. J Natl Cancer Inst 91:1616-1634, 1999 - PubMed
-
- Expert Committee on Tumor-associated Anemia of Chinese Society of Clinical Oncology: Clinical guidelines on tumor associated anemia (version 2015–2016). Chin J Pract Intern Med 35:921-930, 2015
-
- Song ZB, Lu S, Feng JF, et al. : Prevalence and treatment of cancer-related anemia: A nationwide survey in China. China Cancer 28:718-722, 2019
-
- Wright JR, Ung YC, Julian JA, et al. : Randomized, double-blind, placebo-controlled trial of erythropoietin in non-small-cell lung cancer with disease-related anemia. J Clin Oncol 25:1027-1032, 2007 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

