Sexual Health and Quality of Life in Patients With Low-Risk Early-Stage Cervical Cancer: Results From GCIG/CCTG CX.5/SHAPE Trial Comparing Simple Versus Radical Hysterectomy
- PMID: 39353164
- PMCID: PMC11708988
- DOI: 10.1200/JCO.24.00440
Sexual Health and Quality of Life in Patients With Low-Risk Early-Stage Cervical Cancer: Results From GCIG/CCTG CX.5/SHAPE Trial Comparing Simple Versus Radical Hysterectomy
Abstract
Purpose: Simple hysterectomy and pelvic node assessment (SHAPE) is a phase III randomized trial (ClinicalTrials.gov identifier: NCT01658930) reporting noninferiority of simple compared with radical hysterectomy for oncologic outcomes in low-risk cervical cancer. This study presents secondary outcomes of sexual health and quality of life (QOL) of the SHAPE trial.
Methods: Participants were randomly assigned to receive either radical or simple hysterectomy. Sexual health was assessed up to 36 months postoperatively using the Female Sexual Function Index (FSFI) and Female Sexual Distress Scale-Revised and QOL using European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Core 30 and Cervical Cancer-Specific Module (QLQ-CX24) questionnaires.
Results: Among participants with at least one QOL measure, clinical and pathologic characteristics were balanced and with no differences in preoperative baseline scores for sexual health or QOL between groups. FSFI total score met the cutoff for dysfunction up to 6 months (P = .02) in the radical hysterectomy group. Group differences favored simple hysterectomy for FSFI subscales: desire and arousal at 3 months (P ≤ .001) and pain and lubrication up to 12 months (P ≤ .018). Both groups met the cutoff for sexual distress but was higher in radical hysterectomy at 3 months (P = .018). For QLQ-CX24, symptom experience was significantly better up to 24 months (P = .031) and body image better at 3, 24, and 36 months (P ≤ .01) for simple hysterectomy. Sexual-vaginal functioning was significantly better up to 24 months (P ≤ .022) and more sexual activity up to 36 months (P = .024) in the simple hysterectomy arm. Global health status was significantly higher at 36 months for simple hysterectomy (P = .025).
Conclusion: Simple hysterectomy was associated with lower rates of sexual dysfunction than radical hysterectomy, with a lower proportion of women having sustained sexual-vaginal dysfunction. These results further support the benefit of surgical de-escalation for low-risk cervical cancer.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
No other potential conflicts of interest were reported.
Figures
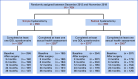

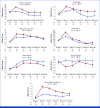
References
-
- Bakker RM, Kenter GG, Creutzberg CL, et al. : Sexual distress and associated factors among cervical cancer survivors: A cross-sectional multicenter observational study. Psychooncology 26:1470-1477, 2017 - PubMed
-
- Kirchheiner K, Smet S, Jürgenliemk-Schulz IM, et al. : Impact of vaginal symptoms and hormonal replacement therapy on sexual outcomes after definitive chemoradiotherapy in patients with locally advanced cervical cancer: Results from the EMBRACE-I study. Int J Radiat Oncol Biol Phys 112:400-413, 2022 - PubMed
-
- Park SY, Bae DS, Nam JH, et al. : Quality of life and sexual problems in disease-free survivors of cervical cancer compared with the general population. Cancer 110:2716-2725, 2007 - PubMed
-
- Bergmark K, Åvall-Lundqvist E, Dickman PW, et al. : Vaginal changes and sexuality in women with a history of cervical cancer. N Engl J Med 340:1383-1389, 1999 - PubMed
-
- Bae H, Park H: Sexual function, depression, and quality of life in patients with cervical cancer. Support Care Cancer 24:1277-1283, 2016 - PubMed

