Synovial vancomycin and meropenem concentrations in periprosthetic joint infection treated by single-stage revision combined with intra-articular infusion
- PMID: 39353609
- PMCID: PMC11444796
- DOI: 10.1302/2046-3758.1310.BJR-2024-0024.R2
Synovial vancomycin and meropenem concentrations in periprosthetic joint infection treated by single-stage revision combined with intra-articular infusion
Abstract
Aims: We aimed to determine the concentrations of synovial vancomycin and meropenem in patients treated by single-stage revision combined with intra-articular infusion following periprosthetic joint infection (PJI), thereby validating this drug delivery approach.
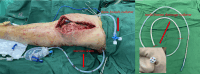
Methods: We included 14 patients with PJI as noted in their medical records between November 2021 and August 2022, comprising eight hip and seven knee joint infections, with one patient experiencing bilateral knee infections. The patients underwent single-stage revision surgery, followed by intra-articular infusion of vancomycin and meropenem (50,000 µg/ml). Synovial fluid samples were collected to assess antibiotic concentrations using high-performance liquid chromatography.
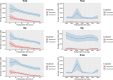
Results: The peak concentrations of vancomycin and meropenem in the joint cavity were observed at one hour post-injection, with mean values of 14,933.9 µg/ml (SD 10,176.3) and 5,819.1 µg/ml (SD 6,029.8), respectively. The trough concentrations at 24 hours were 5,495.0 µg/ml (SD 2,360.5) for vancomycin and 186.4 µg/ml (SD 254.3) for meropenem. The half-life of vancomycin was 6 hours, while that of meropenem ranged between 2 and 3.5 hours. No significant adverse events related to the antibiotic administration were observed.
Conclusion: This method can achieve sustained high antibiotic concentrations within the joint space, exceeding the reported minimum biofilm eradication concentration. Our study highlights the remarkable effectiveness of intra-articular antibiotic infusion in delivering high intra-articular concentrations of antibiotics. The method provided sustained high antibiotic concentrations within the joint cavity, and no severe side-effects were observed. These findings offer evidence to improve clinical treatment strategies. However, further validation is required through studies with larger sample sizes and higher levels of evidence.
© 2024 Zou et al.
Conflict of interest statement
The authors disclose receipt of the following financial or material support for the research, authorship, and/or publication of this article: this work was supported by: Xinjiang Uygur Autonomous Region Natural Science Foundation (No. 2023D01C231); The National Natural Science Foundation of China (No.82260435); Key Laboratory of High Incidence Disease Research in Xinjiang (Xinjiang Medical University), Ministry of Education-Key project (No.2023A01); Major Special Projects of Science and Technology Plan of Xinjiang Uygur Autonomous Region (2022A03011); and Science and Technology Innovation Team Project of Xinjiang Uygur Autonomous Region Science and Technology Department (2023TSYCTD0014).
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources

