Bioreactor-produced iPSCs-derived dopaminergic neuron-containing neural microtissues innervate and normalize rotational bias in a dose-dependent manner in a Parkinson rat model
- PMID: 39353832
- PMCID: PMC11581877
- DOI: 10.1016/j.neurot.2024.e00436
Bioreactor-produced iPSCs-derived dopaminergic neuron-containing neural microtissues innervate and normalize rotational bias in a dose-dependent manner in a Parkinson rat model
Abstract
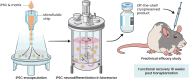
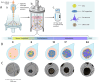
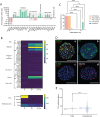
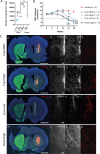
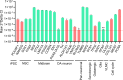
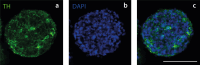
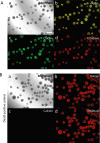
A breadth of preclinical studies now support the rationale of pluripotent stem cell-derived cell replacement therapies to alleviate motor symptoms in Parkinsonian patients. Replacement of the primary dysfunctional cell population in the disease, i.e. the A9 dopaminergic neurons, is the major focus of these therapies. To achieve this, most therapeutical approaches involve grafting single-cell suspensions of DA progenitors. However, most cells die during the transplantation process, as cells face anoïkis. One potential solution to address this challenge is to graft solid preparations, i.e. adopting a 3D format. Cryopreserving such a format remains a major hurdle and is not exempt from causing delays in the time to effect, as observed with cryopreserved single-cell DA progenitors. Here, we used a high-throughput cell-encapsulation technology coupled with bioreactors to provide a 3D culture environment enabling the directed differentiation of hiPSCs into neural microtissues. The proper patterning of these neural microtissues into a midbrain identity was confirmed using orthogonal methods, including qPCR, RNAseq, flow cytometry and immunofluorescent microscopy. The efficacy of the neural microtissues was demonstrated in a dose-dependent manner using a Parkinsonian rat model. The survival of the cells was confirmed by post-mortem histological analysis, characterised by the presence of human dopaminergic neurons projecting into the host striatum. The work reported here is the first bioproduction of a cell therapy for Parkinson's disease in a scalable bioreactor, leading to a full behavioural recovery 16 weeks after transplantation using cryopreserved 3D format.
Keywords: 3D cell format; Cell therapy; Midbrain; Off-the-shelf; Organoid; Regenerative medicine.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Nicolas Prudon reports financial support was provided by TreeFrog Therapeutics. Erwan Bezard reports a relationship with TreeFrog Therapeutics that includes: consulting or advisory. Maxime Feyeux has patent issued to University of Bordeaux. Kevin Alessandri has patent issued to University of Bordeaux. Erwan Bezard has patent issued to University of Bordeaux. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures






References
-
- Olanow C.W., Goetz C.G., Kordower J.H., Stoessl A.J., Sossi V., Brin M.F., et al. A double-blind controlled trial of bilateral fetal nigral transplantation in Parkinson's disease. Ann Neurol. 2003;54:403–414. - PubMed
-
- Freed C.R., Greene P.E., Breeze R.E., Tsai W.Y., DuMouchel W., Kao R., et al. Transplantation of embryonic dopamine neurons for severe Parkinson’s disease. N Engl J Med. 2001;344:710–719. - PubMed
-
- Parmar M., Grealish S., Henchcliffe C. The future of stem cell therapies for Parkinson disease. Nat Rev Neurosci. 2020;21:103–115. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources

