Novel FABP4+C1q+ macrophages enhance antitumor immunity and associated with response to neoadjuvant pembrolizumab and chemotherapy in NSCLC via AMPK/JAK/STAT axis
- PMID: 39353883
- PMCID: PMC11445384
- DOI: 10.1038/s41419-024-07074-x
Novel FABP4+C1q+ macrophages enhance antitumor immunity and associated with response to neoadjuvant pembrolizumab and chemotherapy in NSCLC via AMPK/JAK/STAT axis
Abstract
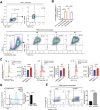
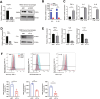
Immune checkpoint inhibitors (ICIs) immunotherapy facilitates new approaches to achieve precision cancer treatment. A growing number of patients with non-small cell lung cancer (NSCLC) have benefited from treatment with neoadjuvant ICIs combined with chemotherapy. However, the mechanisms and associations between the therapeutic efficacy of neoadjuvant pembrolizumab and chemotherapy (NAPC) and macrophage subsets are still unclear. We performed single-cell RNA sequencing (scRNA-seq) and identified a novel FABP4+C1q+ macrophage subtype, which exhibited stronger proinflammatory cytokine production and phagocytic ability. This subtype was found to be more abundant in tumor tissues and lymph nodes of major pathological response (MPR) patients compared to non-MPR patients, and was associated with a good efficacy of NAPC. Multiplex fluorescent immunohistochemical (mIHC) staining was subsequently used to verify our findings. Further mechanistic studies indicated that FABP4 and C1q regulate the expression of proinflammatory cytokines synergistically. In addition, FABP4 and C1q promote fatty acid synthesis, enhance anti-apoptosis ability and phagocytic ability of macrophage via the interaction of AMPK/JAK/STAT axis. This study provides novel insights into the underlying mechanisms and predictive biomarkers of NAPC. Our findings contribute to improving the prognosis of patients with NSCLC by potentially guiding more precise patient selection and treatment strategies. NOVELTY & IMPACT STATEMENTS: We identified a group of macrophages (FABP4+C1q+ macrophages) related to the therapeutic efficacy of neoadjuvant chemoimmunotherapy. FABP4+C1q+ macrophages highly expressed proinflammatory cytokines-related genes and had a strong cytokine production and phagocytic ability. We believe that our study provides a novel insight into the synergistic mechanism of neoadjuvant ICI combined with chemotherapy and may lead to improved clinical outcomes in patients with NSCLC in the future.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49. - PubMed
-
- Schmid P, Cortes J, Pusztai L, McArthur H, Kümmel S, Bergh J, et al. Pembrolizumab plus chemotherapy as neoadjuvant treatment of high-risk, early-stage triple-negative breast cancer: results from the phase 1b open-label, multicohort KEYNOTE-173 study. Ann Oncol. 2020;31:569–81. - PubMed
-
- Zhao X, Huang X, Zhang Z, Li J, Zhou X, Wang C, et al. Final analysis of a phase II trial of neoadjuvant chemoimmunotherapy for locoregionally advanced head and neck squamous cell carcinoma. Oral Oncol. 2024;156:106918. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

