The genetic landscape of basal ganglia and implications for common brain disorders
- PMID: 39353893
- PMCID: PMC11445552
- DOI: 10.1038/s41467-024-52583-0
The genetic landscape of basal ganglia and implications for common brain disorders
Abstract
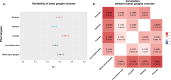
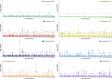
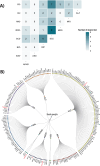
The basal ganglia are subcortical brain structures involved in motor control, cognition, and emotion regulation. We conducted univariate and multivariate genome-wide association analyses (GWAS) to explore the genetic architecture of basal ganglia volumes using brain scans obtained from 34,794 Europeans with replication in 4,808 white and generalization in 5,220 non-white Europeans. Our multivariate GWAS identified 72 genetic loci associated with basal ganglia volumes with a replication rate of 55.6% at P < 0.05 and 87.5% showed the same direction, revealing a distributed genetic architecture across basal ganglia structures. Of these, 50 loci were novel, including exonic regions of APOE, NBR1 and HLAA. We examined the genetic overlap between basal ganglia volumes and several neurological and psychiatric disorders. The strongest genetic overlap was between basal ganglia and Parkinson's disease, as supported by robust LD-score regression-based genetic correlations. Mendelian randomization indicated genetic liability to larger striatal volume as potentially causal for Parkinson's disease, in addition to a suggestive causal effect of greater genetic liability to Alzheimer's disease on smaller accumbens. Functional analyses implicated neurogenesis, neuron differentiation and development in basal ganglia volumes. These results enhance our understanding of the genetic architecture and molecular associations of basal ganglia structure and their role in brain disorders.
© 2024. The Author(s).
Conflict of interest statement
Dr. Andreassen has received a speaker’s honorarium from Lundbeck, Janssen and a consultant for Cortechs.ai. Other authors report no conflicts.
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

