The branched N-glycan of PD-L1 predicts immunotherapy responses in patients with recurrent/metastatic HNSCC
- PMID: 39353912
- PMCID: PMC11445275
- DOI: 10.1038/s41389-024-00532-3
The branched N-glycan of PD-L1 predicts immunotherapy responses in patients with recurrent/metastatic HNSCC
Abstract
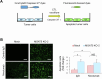
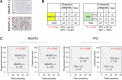
Immunotherapy has revolutionized cancer treatment, but the lack of a reliable predictive biomarker for treatment response remains a challenge. Alpha-1,6-Mannosylglycoprotein 6-β-N-Acetylglucosaminyltransferase 5 (MGAT5) is a key regulator of complex N-glycan synthesis, and its dysregulation is associated with cancer progression. The lectin Phaseolus vulgaris leukoagglutinin (PHA-L) specifically binds to mature MGAT5 products. Previous studies have indicated elevated PHA-L staining in head and neck squamous cell carcinoma (HNSCC), which implies increased activity of MGAT5. However, the specific role of MGAT5 in HNSCC remains unclear. In this study, we found significantly higher PHA-L staining and MGAT5 expression in HNSCC tumors compared to adjacent non-tumor tissues. Using a mass spectrometry (MS)-based glycoproteomic approach, we identified 163 potential protein substrates of MGAT5. Functional analysis revealed that protein substrates of MGAT5 regulated pathways related to T cell proliferation and activation. We further discovered that PD-L1 was among the protein substrates of MGAT5, and the expression of MGAT5 protected tumor cells from cytotoxic T lymphocyte (CTL) killing. Treatment of nivolumab alleviated the protective effects of MGAT5 on CTL activity. Consistently, patients with MGAT5-positive tumors showed improved responses to immunotherapy compared to those with MGAT5-negative tumors. Using purified PD-L1 from HNSCC cells and a glycoproteomic approach, we further deciphered that the N35 and N200 sites carry the majority of complex N-glycans on PD-L1. Our findings highlight the critical role of MGAT5-mediated branched N-glycans on PD-L1 in modulating the interaction with the immune checkpoint receptor PD-1. Consequently, we propose that MGAT5 could serve as a biomarker to predict patients' responses to anti-PD-1 therapy. Furthermore, targeting the branched N-glycans at N35 and N200 of PD-L1 may lead to the development of novel diagnostic and therapeutic approaches.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Mody MD, Rocco JW, Yom SS, Haddad RI, Saba NF. Head and neck cancer. Lancet. 2021;398:2289–99. - PubMed
-
- Burtness B, Harrington KJ, Greil R, Soulières D, Tahara M, de Castro G Jr., et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet. 2019;394:1915–28. - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

