Inhibition of poly(ADP-Ribosyl)ation reduced vascular smooth muscle cells loss and improves aortic disease in a mouse model of human accelerated aging syndrome
- PMID: 39353941
- PMCID: PMC11448498
- DOI: 10.1038/s41419-024-07078-7
Inhibition of poly(ADP-Ribosyl)ation reduced vascular smooth muscle cells loss and improves aortic disease in a mouse model of human accelerated aging syndrome
Abstract
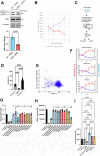
Hutchinson-Gilford progeria syndrome (HGPS) is an extremely rare genetic disorder associated with features of accelerated aging. HGPS is an autosomal dominant disease caused by a de novo mutation of LMNA gene, encoding A-type lamins, resulting in the truncated form of pre-lamin A called progerin. While asymptomatic at birth, patients develop symptoms within the first year of life when they begin to display accelerated aging and suffer from growth retardation, and severe cardiovascular complications including loss of vascular smooth muscle cells (VSMCs). Recent works reported the loss of VSMCs as a major factor triggering atherosclerosis in HGPS. Here, we investigated the mechanisms by which progerin expression leads to massive VSMCs loss. Using aorta tissue and primary cultures of murine VSMCs from a mouse model of HGPS, we showed increased VSMCs death associated with increased poly(ADP-Ribosyl)ation. Poly(ADP-Ribosyl)ation is recognized as a post-translational protein modification that coordinates the repair at DNA damage sites. Poly-ADP-ribose polymerase (PARP) catalyzes protein poly(ADP-Ribosyl)ation by utilizing nicotinamide adenine dinucleotide (NAD+). Our results provided the first demonstration linking progerin accumulation, augmented poly(ADP-Ribosyl)ation and decreased nicotinamide adenine dinucleotide (NAD+) level in VSMCs. Using high-throughput screening on VSMCs differentiated from iPSCs from HGPS patients, we identified a new compound, trifluridine able to increase NAD+ levels through decrease of PARP-1 activity. Lastly, we demonstrate that trifluridine treatment in vivo was able to alleviate aortic VSMCs loss and clinical sign of progeria, suggesting a novel therapeutic approach of cardiovascular disease in progeria.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- De Sandre-Giovannoli A, Bernard R, Cau P, Navarro C, Amiel J, Boccaccio I, et al. Lamin A truncation in Hutchinson-Gilford progeria. Science. 2003;300:2055. - PubMed
-
- Lin F, Worman HJ. Structural organization of the human gene encoding nuclear lamin A and nuclear lamin C. J Biol Chem. 1993;268:16321–6. - PubMed
-
- Nigg EA, Kitten GT, Vorburger K. Targeting lamin proteins to the nuclear envelope: the role of CaaX box modifications. Biochem Soc Trans. 1992;20:500–4. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

