Superior metabolic improvement of polycystic ovary syndrome traits after GLP1-based multi-agonist therapy
- PMID: 39353946
- PMCID: PMC11445520
- DOI: 10.1038/s41467-024-52898-y
Superior metabolic improvement of polycystic ovary syndrome traits after GLP1-based multi-agonist therapy
Abstract
Polycystic ovary syndrome (PCOS) is a heterogeneous condition, defined by oligo-/anovulation, hyper-androgenism and/or polycystic ovaries. Metabolic complications are common in patients suffering PCOS, including obesity, insulin resistance and type-2 diabetes, which severely compromise the clinical course of affected women. Yet, therapeutic options remain mostly symptomatic and of limited efficacy for the metabolic and reproductive alterations of PCOS. We report here the hormonal, metabolic and gonadal responses to the glucagon-like peptide-1 (GLP1)-based multi-agonists, GLP1/Estrogen (GLP1/E), GLP1/gastric inhibitory peptide (GLP1/GIP) and GLP1/GIP/Glucagon, in two mouse PCOS models, with variable penetrance of metabolic and reproductive traits, and their comparison with metformin. Our data illustrate the superior efficacy of GLP1/E vs. other multi-agonists and metformin in the management of metabolic complications of PCOS; GLP1/E ameliorates also ovarian cyclicity in an ovulatory model of PCOS, without direct estrogenic uterotrophic effects. In keeping with GLP1-mediated brain targeting, quantitative proteomics reveals changes in common and distinct hypothalamic pathways in response to GLP1/E between the two PCOS models, as basis for differential efficiency. Altogether, our data set the basis for the use of GLP1-based multi-agonists, and particularly GLP1/E, in the personalized management of PCOS.
© 2024. The Author(s).
Conflict of interest statement
B.F., J.D.D., and B.Y. are former employees of Novo Nordisk. T.D.M. received funding from Novo Nordisk and speaking fees within the last 3 years from Novo Nordisk, Eli Lilly, Astra Zeneca, and Berlin Chemie AG. M.H.T. is a member of the SAB of ERX Pharmaceuticals, Inc. (last SAB meeting in 2019). Since 2018, he has served as the CSO and CEO of Helmholtz Munich (an academic research center with ca. 2500 scientists and staff). Helmholtz Munich is involved in numerous collaborations with a multitude of companies and institutions, worldwide. Cooperation, at times, involves research funding and includes (but are not limited to) interactions with e.g., Novo Nordisk, Boehringer Ingelheim, Roche Diagnostics, Arbormed, SCG Cell Therapy, and others. None of the above funding sources were involved in the preparation of this paper. M.T.S. has participated in the last 3 years in projects from Novo Nordisk and collaborated via grant agreements with the company Oxo-Life Ltd. (Barcelona, Spain), addressing therapeutic strategies for PCOS, although no connection exists between these projects/agreements and the present work. The rest of the authors have no conflict of interest to disclose in relation to the contents of this work.
Figures




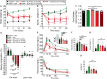





References
Publication types
MeSH terms
Substances
Grants and funding
- BFU2017-83934-P/Ministry of Economy and Competitiveness | Agencia Estatal de Investigación (Spanish Agencia Estatal de Investigación)
- PID2020-118660GB-I00/Ministry of Economy and Competitiveness | Agencia Estatal de Investigación (Spanish Agencia Estatal de Investigación)
- PIE14-00005/Ministry of Economy and Competitiveness | Instituto de Salud Carlos III (Institute of Health Carlos III)
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

