The activation of the Notch signaling pathway by UBE2C promotes the proliferation and metastasis of hepatocellular carcinoma
- PMID: 39353974
- PMCID: PMC11445553
- DOI: 10.1038/s41598-024-72714-3
The activation of the Notch signaling pathway by UBE2C promotes the proliferation and metastasis of hepatocellular carcinoma
Abstract
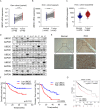
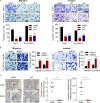
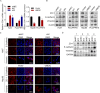
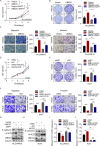
UBE2C, a ubiquitin-conjugating enzyme, functions as an oncogene in different types of human cancers. Nonetheless, the exact influence of UBE2C on the development of HCC via regulation of ubiquitination remains uncertain. Here, we found that UBE2C displayed elevated levels of expression in HCC and was associated with an unfavorable prognosis, as evidenced by the analysis of the TCGA database and the examination of clinical specimens. The role of UBE2C in HCC revealed its ability to promote the growth and metastasis of HCC. Mechanistically, UBE2C activated Notch signaling, as evidenced by the upregulation of N1ICD and Hes1, crucial components of the Notch pathway, and activation of the RBP-JK luciferase reporter by UBE2C. Finally, rescue experiments demonstrated that the oncogenic role of UBE2C was eliminated through treatment with the Notch inhibitor DAPT, while overexpression of N1ICD alleviated the anticarcinogenic impact of knockdown of UBE2C. Altogether, the results of our study indicate that UBE2C plays a role in the activation of Notch signaling and could potentially serve as a viable target for therapeutic interventions in HCC.
Keywords: EMT; Notch signaling; Ubiquitination.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

