Spatiotemporal control of neutrophil fate to tune inflammation and repair for myocardial infarction therapy
- PMID: 39353987
- PMCID: PMC11445496
- DOI: 10.1038/s41467-024-52812-6
Spatiotemporal control of neutrophil fate to tune inflammation and repair for myocardial infarction therapy
Abstract
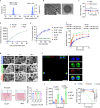
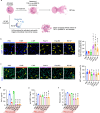
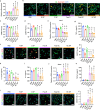
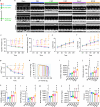
Neutrophils are critical mediators of both the initiation and resolution of inflammation after myocardial infarction (MI). Overexuberant neutrophil signaling after MI exacerbates cardiomyocyte apoptosis and cardiac remodeling while neutrophil apoptosis at the injury site promotes macrophage polarization toward a pro-resolving phenotype. Here, we describe a nanoparticle that provides spatiotemporal control over neutrophil fate to both stymie MI pathogenesis and promote healing. Intravenous injection of roscovitine/catalase-loaded poly(lactic-co-glycolic acid) nanoparticles after MI leads to nanoparticle uptake by circulating neutrophils migrating to the infarcted heart. Activated neutrophils at the infarcted heart generate reactive oxygen species, triggering intracellular release of roscovitine, a cyclin-dependent kinase inhibitor, from the nanoparticles, thereby inducing neutrophil apoptosis. Timely apoptosis of activated neutrophils at the infarcted heart limits neutrophil-driven inflammation, promotes macrophage polarization toward a pro-resolving phenotype, and preserves heart function. Modulating neutrophil fate to tune both inflammatory and reparatory processes may be an effective strategy to treat MI.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- Reed, G. W., Rossi, J. E. & Cannon, C. P. Acute myocardial infarction. Lancet389, 197–210 (2017). - PubMed
-
- Anderson, J. L. & Morrow, D. A. Acute myocardial infarction. N. Engl. J. Med.376, 2053–2064 (2017). - PubMed
-
- Vafadarnejad, E. et al. Dynamics of cardiac neutrophil diversity in murine myocardial infarction. Circ. Res.127, e232–e249 (2020). - PubMed
-
- Soehnlein, O., Steffens, S., Hidalgo, A. & Weber, C. Neutrophils as protagonists and targets in chronic inflammation. Nat. Rev. Immunol.17, 248–261 (2017). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

