The role of TERT C228T and KDM6A alterations and TME in NMIBC treated with BCG
- PMID: 39353991
- PMCID: PMC11445404
- DOI: 10.1038/s41698-024-00725-4
The role of TERT C228T and KDM6A alterations and TME in NMIBC treated with BCG
Abstract
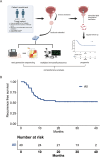
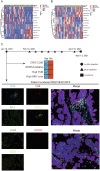
We aimed to investigate the genomic and tumor microenvironmental (TME) profiles in non-muscle invasive bladder cancer (NMIBC) and explore potential predictive markers for Bacillus Calmette-Guérin (BCG) treatment response in high-risk NMIBC patients (according to European Association of Urology (EAU) risk stratification). 40 patients with high-risk NMIBC (cTis-T1N0M0) who underwent en bloc resection followed by BCG instillation were retrospectively enrolled. Surgical samples were subjected to Next Generation Sequencing (NGS) and multiplex immunofluorescence (mIF) assay. Genomic profiling revealed high prevalences of alterations in TERT (55%), KDM6A (32.5%), FGFR3(30%), PIK3CA (30%), TP53(27.5%) and ARID1A (20%). TME analysis showed different proportions of macrophages, NK cells, T cells subsets in tumoral and stromal compartment. Multivariate analysis identified TERT C228T and alteration in KDM6A as two independent factors associated with inferior RFS. The study comprehensively depicted the genomic and TME profiles in NMIBC and identified potential predictive biomarkers for BCG treatment.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Babjuk, M. et al. European Association of Urology Guidelines on Non-muscle-invasive Bladder Cancer (Ta, T1, and Carcinoma in Situ). Eur. Urol.81, 75–94 (2022). - PubMed
-
- Antoni, S. et al. Bladder cancer incidence and mortality: a global overview and recent trends. Eur. Urol.71, 96–108 (2017). - PubMed
-
- Lobo, N. et al. Epidemiology, screening, and prevention of bladder cancer. Eur. Urol. Oncol.5, 628–639 (2022). - PubMed
-
- Li, Z., Zhou, Z., Cui, Y. & Zhang, Y. Systematic review and meta-analysis of randomized controlled trials of perioperative outcomes and prognosis of transurethral en-bloc resection vs. conventional transurethral resection for non-muscle-invasive bladder cancer. Int. J. Surg.104, 106777 (2022). - PubMed
-
- Lenis, A. T., Lec, P. M., Chamie, K. & Mshs, M. D. Bladder cancer: a review. Jama324, 1980–1991 (2020). - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

