Molecular landscape of ERBB2 alterations in 3000 advanced NSCLC patients
- PMID: 39354054
- PMCID: PMC11445497
- DOI: 10.1038/s41698-024-00720-9
Molecular landscape of ERBB2 alterations in 3000 advanced NSCLC patients
Abstract
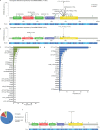
ERBB2 (HER2) represents a newly recognized actionable oncogenic driver in non-small cell lung cancer (NSCLC), with approved targeted therapy available. Understanding the landscape of ERBB2 alterations and co-occurring mutations is essential for guiding treatment decisions. We conducted an analysis involving 3000 NSCLC patients with all types of ERBB2 alterations, drawn from two extensive retrospective cohorts: 1281 from Geneplus (Chinese) and 1719 from Guardant360 (the United States, US). The incidence of all types of ERBB2 alterations was found to be 5.6% in the Chinese group and 5.2% in the US group. In both cohorts, among oncogenic alterations of ERBB2, exon 20 insertion Y772_A775dupYVMA was the most frequent alteration (58% vs 41.6% in the Chinese vs the US), followed by G776delinsVC/LC/VV/IC (10.7% vs 9.7%), and S310X (10.5% vs 15.4%). EGFR ex20 insertions were identified in the A767-V774 region, whereas ERBB2 ex20 insertions were observed in the Y772-P780 region. Notably, EGFR ex20 insertions exhibited greater insertion diversity. Clinical characteristics of EGFR and ERBB2 ex20 NSCLC were similar, characterized by low tumor mutation burden (TMB), a predominant never-smoker population, and a majority of lung adenocarcinoma cases.
© 2024. The Author(s).
Conflict of interest statement
L.M.D. is employee and stockholder of Guardant Health. Y.X. and R.C. are employee of Geneplus-Beijing. S.H. received consulting fees from Guardant health and AstraZeneca. M.N. receives royalties and licensing fees from Spectrum Pharmaceuticals. J.V.H. reports receiving advisory/consulting fees from AstraZeneca, Boehringer-Ingeheim, Catalyst, Genentech, GlaxoSmithKline, Guardant Health, Foundation Medicine, Hengrui Therapeutics, Eli Lilly, Novartis, Spectrum, Sanofi, Takeda Pharmaceuticals, Mirati Therapeutics, Bristiol-Myers Squibb, BrightPath Biotherapeutics, Janssen Global Services, Nexus Health Systems, EMD Serono, Pneuma Respiratory, Kairos Venture Investments, Leads Biolabs, RefleXion, and research funding from GlaxoSmithKline, AstraZeneca, Spectrum, all outside of the submitted work. J.Z. reports grants from Merck, grants and personal fees from Johnson and Johnson and Novartis, and personal fees from Bristol Myers Squibb, AstraZeneca, GenePlus, Innovent, and Hengrui outside the submitted work. X.L. receives consulting/advisory fees from EMD Serono (Merck KGaA), AstraZeneca, Spectrum Pharmaceutics, Novartis, Eli Lilly, Boehringer Ingelheim, Hengrui Therapeutics, Janssen, Blueprint Medicines, Regeneron, Sensei Biotherapeutics, Abion, Pinetree Therapeutics and Abbvie, and Research Funding from Eli Lilly, EMD Serono, ArriVent, Teligene, Regeneron, Blackdiamond, and Boehringer Ingelheim. The other authors declare no competing interests in the submitted work.
Figures



References
-
- Friedlaender, A. et al. EGFR and HER2 exon 20 insertions in solid tumours: from biology to treatment. Nat. Rev. Clin. Oncol.19, 51–69 (2022). - PubMed
-
- Shigematsu, H. et al. Somatic mutations of the HER2 kinase domain in lung adenocarcinomas. Cancer Res. 65, 1642–6, (2005). - PubMed
-
- Tomizawa, K. et al. Prognostic and predictive implications of HER2/ERBB2/neu gene mutations in lung cancers. Lung Cancer74, 139–44, (2011). - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

