Combinatorial design of siloxane-incorporated lipid nanoparticles augments intracellular processing for tissue-specific mRNA therapeutic delivery
- PMID: 39354147
- PMCID: PMC12207990
- DOI: 10.1038/s41565-024-01747-6
Combinatorial design of siloxane-incorporated lipid nanoparticles augments intracellular processing for tissue-specific mRNA therapeutic delivery
Abstract
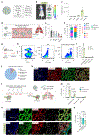
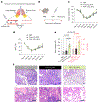
Systemic delivery of messenger RNA (mRNA) for tissue-specific targeting using lipid nanoparticles (LNPs) holds great therapeutic potential. Nevertheless, how the structural characteristics of ionizable lipids (lipidoids) impact their capability to target cells and organs remains unclear. Here we engineered a class of siloxane-based ionizable lipids with varying structures and formulated siloxane-incorporated LNPs (SiLNPs) to control in vivo mRNA delivery to the liver, lung and spleen in mice. The siloxane moieties enhance cellular internalization of mRNA-LNPs and improve their endosomal escape capacity, augmenting their mRNA delivery efficacy. Using organ-specific SiLNPs to deliver gene editing machinery, we achieve robust gene knockout in the liver of wild-type mice and in the lungs of both transgenic GFP and Lewis lung carcinoma (LLC) tumour-bearing mice. Moreover, we showed effective recovery from viral infection-induced lung damage by delivering angiogenic factors with lung-targeted Si5-N14 LNPs. We envision that our SiLNPs will aid in the clinical translation of mRNA therapeutics for next-generation tissue-specific protein replacement therapies, regenerative medicine and gene editing.
© 2024. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
Competing interests: L.X. and M.J.M. are inventors on a patent filed by the Trustees of the University of Pennsylvania (International Patent Application No. PCT/US23/66564) describing the lipid nanoparticle technology in this study. J.M.W. is a paid advisor to and holds equity in iECURE, Passage Bio and the Center for Breakthrough Medicines (CBM). He also holds equity in the former G2 Bio asset companies and Ceva Santé Animale. He has sponsored research agreements with Alexion Pharmaceuticals, Amicus Therapeutics, CBM, Ceva Santé Animale, Elaaj Bio, FA212, Foundation for Angelman Syndrome Therapeutics, former G2 Bio asset companies, iECURE and Passage Bio, which are licensees of Penn Technology. J.M.W., L.W. and C.C.W are inventors on patents that have been licensed to various biopharmaceutical companies and for which they may receive payments. D.W. is named on patents that describe the use of nucleoside-modified mRNA as a platform to deliver therapeutic proteins and vaccines. M.J.M., D.W. and M.-G.A. are also named on patents describing the use of lipid nanoparticles and lipid compositions for nucleic acid delivery. The other authors declare no competing interests.
Figures






References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

