Human DUS1L catalyzes dihydrouridine modification at tRNA positions 16/17, and DUS1L overexpression perturbs translation
- PMID: 39354220
- PMCID: PMC11445529
- DOI: 10.1038/s42003-024-06942-8
Human DUS1L catalyzes dihydrouridine modification at tRNA positions 16/17, and DUS1L overexpression perturbs translation
Abstract
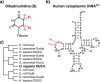
Human cytoplasmic tRNAs contain dihydrouridine modifications at positions 16 and 17 (D16/D17). The enzyme responsible for D16/D17 formation and its cellular roles remain elusive. Here, we identify DUS1L as the human tRNA D16/D17 writer. DUS1L knockout in the glioblastoma cell lines LNZ308 and U87 causes loss of D16/D17. D formation is reconstituted in vitro using recombinant DUS1L in the presence of NADPH or NADH. DUS1L knockout/overexpression in LNZ308 cells shows that DUS1L supports cell growth. Moreover, higher DUS1L expression in glioma patients is associated with poorer prognosis. Upon vector-mediated DUS1L overexpression in LNZ308 cells, 5' and 3' processing of precursor tRNATyr(GUA) is inhibited, resulting in a reduced mature tRNATyr(GUA) level, reduced translation of the tyrosine codons UAC and UAU, and reduced translational readthrough of the near-cognate stop codons UAA and UAG. Moreover, DUS1L overexpression increases the amounts of several D16/D17-containing tRNAs and total cellular translation. Our study identifies a human dihydrouridine writer, providing the foundation to study its roles in health and disease.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- Crick, F. On protein synthesis. Symp. Soc. Exp. Biol.12, 138–163 (1958). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

