Structure of Fanzor2 reveals insights into the evolution of the TnpB superfamily
- PMID: 39354233
- PMCID: PMC11832414
- DOI: 10.1038/s41594-024-01394-4
Structure of Fanzor2 reveals insights into the evolution of the TnpB superfamily
Abstract
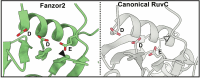
RNA-guided endonucleases, once thought to be exclusive to prokaryotes, have been recently identified in eukaryotes and are called Fanzors. They are classified into two clades, Fanzor1 and Fanzor2. Here we present the cryo-electron microscopy structure of Acanthamoeba polyphaga mimivirus Fanzor2, revealing its ωRNA architecture, active site and features involved in transposon-adjacent motif recognition. A comparison to Fanzor1 and TnpB structures highlights divergent evolutionary paths, advancing our understanding of RNA-guided endonucleases.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures










References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

