Efficacy and safety of shorter multidrug-resistant or rifampicin-resistant tuberculosis regimens: a network meta-analysis
- PMID: 39354416
- PMCID: PMC11443784
- DOI: 10.1186/s12879-024-09960-3
Efficacy and safety of shorter multidrug-resistant or rifampicin-resistant tuberculosis regimens: a network meta-analysis
Abstract
Background: Drug-resistant tuberculosis (DR-TB) remains a threat to public health. Shorter regimens have been proposed as potentially valuable treatments for multidrug or rifampicin resistant tuberculosis (MDR/RR-TB). We undertook a systematic review and network meta-analysis to evaluate the efficacy and safety of shorter MDR/RR-TB regimens.
Methods: We searched PubMed/MEDLINE, Cochrane Center for Clinical Trials (CENTRAL), Scopus, ClinicalTrials.gov, WHO International Clinical Trials Registry Platform, US Food and Drug Administration, and Chinese Clinical Trial Registry for primary articles published from 2013 to July 2023. Favorable (cured and treatment completed) and unfavorable (treatment failure, death, loss to follow-up, and culture conversion) outcomes were assessed as the main efficacy outcomes, while adverse events were assessed as the safety outcomes. The network meta-analysis was performed using R Studio version 4.3.1 and the Netmeta package. The study protocol adhered to the PRISMA-NMA guidelines and was registered in PROSPERO (CRD42023434050).
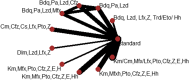
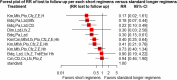
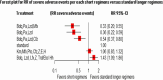
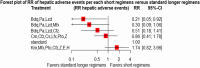
Result: We included 11 eligible studies (4 randomized control trials and 7 cohorts) that enrolled 3,548 patients with MDR/RR-TB. Treatment with a 6-month combination of BdqLzdLfxZTrd/Eto/H had two times more favorable outcomes [RR 2.2 (95% CI 1.22, 4.13), P = 0.0094], followed by a 9-11 month combination of km/CmMfx/LfxPtoCfzZEHh [RR1.67 (95% CI 1.45, 1.92), P < 0.001] and a 6-month BdqPaLzdMfx [RR 1.64 (95% CI 1.24, 2.16), P < 0.0005] compared to the standard longer regimens. Treatment with 6 months of BdqPaLzdMfx [RR 0.33 (95% CI 0.2, 0.55), P < 0.0001] had a low risk of severe adverse events, followed by 6 months of BdqPaLzd [RR 0.36 (95% CI 0.22, 0.59), P ≤ 0.001] and BdqPaLzdCfz [RR 0.54 (95% CI 0.37, 0.80), P < 0.0001] than standard of care.
Conclusion: Treatment of patients with RR/MDR-TB using shorter regimens of 6 months BdqLzdLfxZTrd/Eto/H, 9-11 months km/CmMfx/LfxPtoCfzZEHh, and 6 months BdqPaLzdMfx provides significantly higher cure and treatment completion rates compared to the standard longer MDR/RR-TB. However, 6BdqPaLzdMfx, 6BdqPaLzd, and 6BdqPaLzdCfz short regimens are significantly associated with decreased severity of adverse events. The findings are in support of the current WHO-recommended 6-month shorter regimens.
Keywords: Multidrug-resistant tuberculosis (MDR-TB); Network meta-analysis; Rifampicin-resistant tuberculosis (RR-TB); Short- term regimens; Systematic review.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


















References
-
- Global tuberculosis report 2023.World Health Organization. 2023.https://www.who.int/publications-detail-redirect/9789240083851. Cited 2023 Nov 13.
-
- Migliori GB, Tiberi S. WHO drug-resistant TB guidelines 2022: what is new? Int J Tuberc Lung Dis. 2022;26(7):590–1. - PubMed
-
- Organization WH. WHO consolidated guidelines on tuberculosis: module 4: treatment: drug-susceptible tuberculosis treatment. In: WHO consolidated guidelines on tuberculosis: module 4: treatment: drug-susceptible tuberculosis treatment. 2022. - PubMed
-
- WHO consolidated guidelines on drug-resistant tuberculosis treatment. Geneva: World Health Organization. 2019. In. https://www.ncbi.nlm.nih.gov/books/NBK539518/table/ch1.tab2/. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

