Weekly carboplatin plus paclitaxel chemotherapy in advanced melanoma patients resistant to anti-PD-1 inhibitors: a retrospective, monocentric experience
- PMID: 39354418
- PMCID: PMC11446135
- DOI: 10.1186/s12885-024-12961-9
Weekly carboplatin plus paclitaxel chemotherapy in advanced melanoma patients resistant to anti-PD-1 inhibitors: a retrospective, monocentric experience
Abstract
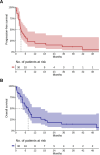
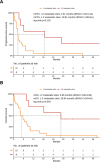
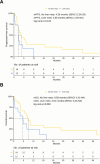
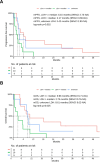
Immunotherapy with anti-PD-1 antibodies significantly improved the prognosis in advanced melanoma patients, but most of them develop primary or secondary resistance to the treatment. In this study, we evaluated efficacy and safety of a chemotherapy regimen with weekly carboplatin plus paclitaxel (wCP) in patients previously treated with anti-PD-1 antibodies. We retrospectively identified 30 patients with advanced melanoma treated at our Institute over the last eight years with wCP. The co-primary endpoints of the study were overall survival (OS) and progression-free survival (PFS). In addition, we evaluated treatment tolerability. For this patient cohort, median PFS and OS were 3.25 and 7.69 months, respectively. All included patients had previously received anti-PD-1 immunotherapy, most of them had ECOG PS 0-1, and only 5 patients had a BRAF V600 mutation. In univariable analysis, we observed shorter OS in patients with > 2 involved metastatic sites, superficial spreading histology, and serum lactate dehydrogenase (LDH) values above the median. Liver metastases were associated with worse outcomes, while radiotherapy treatment of brain metastases was associated with improved OS. However, in a multivariable Cox regression model, only LDH above the median, superficial spreading histology, and female sex were significantly associated with worse OS. We reported grade 3 and 4 treatment-related toxicities in 4 and 0 patients, respectively. In conclusion, chemotherapy with wCP is a valid palliative treatment in advanced melanoma who progressed with anti-PD-1 antibodies.
Keywords: Immunoresistance; Melanoma; Palliative treatment; Platinum-based chemotherapy.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
FdG has been a speaker at BMS, and Novartis conference. PM had a consultant/advisory role for BMS, ROCHE Genentech, MSD, Novartis, AMGEN, Merck Serono, Pierre Fabre, INCYTE. The two authors declare that this research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. All other authors declare no conflict of interest.
Figures
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

