Application of a single-cell-RNA-based biological-inspired graph neural network in diagnosis of primary liver tumors
- PMID: 39354613
- PMCID: PMC11445937
- DOI: 10.1186/s12967-024-05670-1
Application of a single-cell-RNA-based biological-inspired graph neural network in diagnosis of primary liver tumors
Abstract
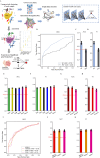
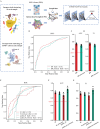
Single-cell technology depicts integrated tumor profiles including both tumor cells and tumor microenvironments, which theoretically enables more robust diagnosis than traditional diagnostic standards based on only pathology. However, the inherent challenges of single-cell RNA sequencing (scRNA-seq) data, such as high dimensionality, low signal-to-noise ratio (SNR), sparse and non-Euclidean nature, pose significant obstacles for traditional diagnostic approaches. The diagnostic value of single-cell technology has been largely unexplored despite the potential advantages. Here, we present a graph neural network-based framework tailored for molecular diagnosis of primary liver tumors using scRNA-seq data. Our approach capitalizes on the biological plausibility inherent in the intercellular communication networks within tumor samples. By integrating pathway activation features within cell clusters and modeling unidirectional inter-cellular communication, we achieve robust discrimination between malignant tumors (including hepatocellular carcinoma, HCC, and intrahepatic cholangiocarcinoma, iCCA) and benign tumors (focal nodular hyperplasia, FNH) by scRNA data of all tissue cells and immunocytes only. The efficacy to distinguish iCCA from HCC was further validated on public datasets. Through extending the application of high-throughput scRNA-seq data into diagnosis approaches focusing on integrated tumor microenvironment profiles rather than a few tumor markers, this framework also sheds light on minimal-invasive diagnostic methods based on migrating/circulating immunocytes.
Keywords: Diagnostic model; Graph neural network; Primary liver tumors; Single-cell transcriptome; Tumor microenvironment.
© 2024. The Author(s).
Conflict of interest statement
All authors claim that there is no conflict of interest.
Figures


References
-
- National Comprehensive Cancer Network Guidelines for Hepatobiliary Cancers. (Version 1.2022). https://www.nccn.org/guidelines/guidelines-process/transparency-process-.... Published 2022. Accessed May 4, 2023, 2023.
-
- Hu C, Xia T, Cui Y, et al. Trustworthy multi-phase liver tumor segmentation via evidence-based uncertainty. Eng Appl Artif Intell. 2024;133:108289. - DOI
MeSH terms
Substances
Grants and funding
- 22XD1402700/Program of Shanghai Academic Research Leader
- XHLHGG202103/Key Disease Joint Research Program of Xuhui District
- 2019YFC1316000/the National Key Research and Development Program of China
- 82273234/the National Natural Science Foundation of China
- GDXZ-08-05/Beijing Mutual Care Public Welfare Foundation
- No. SZSM202003009/Sanming Project of Medicine in Shenzhen
- the Outstanding Resident Clinical Postdoctoral Program of Zhongshan Hospital Affiliated to Fudan University/the Outstanding Resident Clinical Postdoctoral Program of Zhongshan Hospital Affiliated to Fudan University
- Youth Fund of Zhongshan Hospital Affiliated to Fudan University/Youth Fund of Zhongshan Hospital Affiliated to Fudan University
LinkOut - more resources
Full Text Sources
Medical

