Dysregulated nicotinamide adenine dinucleotide metabolome in patients hospitalized with COVID-19
- PMID: 39354697
- PMCID: PMC11634700
- DOI: 10.1111/acel.14326
Dysregulated nicotinamide adenine dinucleotide metabolome in patients hospitalized with COVID-19
Abstract
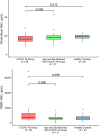
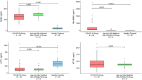
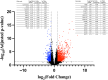
Nicotinamide adenine dinucleotide (NAD+) depletion has been postulated as a contributor to the severity of COVID-19; however, no study has prospectively characterized NAD+ and its metabolites in relation to disease severity in patients with COVID-19. We measured NAD+ and its metabolites in 56 hospitalized patients with COVID-19 and in two control groups without COVID-19: (1) 31 age- and sex-matched adults with comorbidities, and (2) 30 adults without comorbidities. Blood NAD+ concentrations in COVID-19 group were only slightly lower than in the control groups (p < 0.05); however, plasma 1-methylnicotinamide concentrations were significantly higher in patients with COVID-19 (439.7 ng/mL, 95% CI: 234.0, 645.4 ng/mL) than in age- and sex-matched controls (44.5 ng/mL, 95% CI: 15.6, 73.4) and in healthy controls (18.1 ng/mL, 95% CI 15.4, 20.8; p < 0.001 for each comparison). Plasma nicotinamide concentrations were also higher in COVID-19 group and in controls with comorbidities than in healthy control group. Plasma concentrations of 2-methyl-2-pyridone-5-carboxamide (2-PY), but not NAD+, were significantly associated with increased risk of death (HR = 3.65; 95% CI 1.09, 12.2; p = 0.036) and escalation in level of care (HR = 2.90, 95% CI 1.01, 8.38, p = 0.049). RNAseq and RTqPCR analyses of PBMC mRNA found upregulation of multiple genes involved in NAD+ synthesis as well as degradation, and dysregulation of NAD+-dependent processes including immune response, DNA repair, metabolism, apoptosis/autophagy, redox reactions, and mitochondrial function. Blood NAD+ concentrations are modestly reduced in COVID-19; however, NAD+ turnover is substantially increased with upregulation of genes involved in both NAD+ biosynthesis and degradation, supporting the rationale for NAD+ augmentation to attenuate disease severity.
Keywords: 1‐methylnicotinamide; 2PY; NAD+ augmentation in COVID‐19; NAD+ metabolites; NAD+ turnover; SARS‐CoV‐2 infection; nicotinamide.
© 2024 The Author(s). Aging Cell published by Anatomical Society and John Wiley & Sons Ltd.
Conflict of interest statement
Dr. Bhasin's institution has received grants from NIA, NICHD‐NCMRR, DoD, AbbVie, Besins, FPT, and Metro International Biotech, on which Dr. Bhasin serves as the principal investigator, and consulting fees from Besins, Novartis, and Varsenis. These conflicts are overseen by the Office of Industry Interaction of Mass General Brigham in accordance with institutional rules and regulations. DJL, PS, PL, and SL are employed by Metro International Biotech. MMnt receives support from the Merck Investigators Studies Program and the Claude D. Pepper OAIC. MMnt is also the Editor‐in‐Chief of Aging Cell and was blinded to the review of this manuscript. RV, BW, KP, YS, NL, MMig, JC, LW, LP, YM, and AB have no conflicts of interest to declare.
Figures


References
-
- Altay, O. , Arif, M. , Li, X. , Yang, H. , Aydin, M. , Alkurt, G. , Kim, W. , Akyol, D. , Zhang, C. , Dinler‐Doganay, G. , Turkez, H. , Shoaie, S. , Nielsen, J. , Boren, J. , Olmuscelik, O. , Doganay, L. , Uhlen, M. , & Mardinoglu, A. (2021). Combined metabolic activators accelerates recovery in mild‐to‐moderate COVID‐19. Advanced Science, 8, e2101222. - PMC - PubMed
-
- Bar, A. , Olkowicz, M. , Tyrankiewicz, U. , Kus, E. , Jasinski, K. , Smolenski, R. T. , Skorka, T. , & Chlopicki, S. (2017). Functional and biochemical endothelial profiling in vivo in a murine model of endothelial dysfunction; comparison of effects of 1‐Methylnicotinamide and angiotensin‐converting enzyme inhibitor. Frontiers in Pharmacology, 8, 183. - PMC - PubMed
-
- Bhasin, S. , Seals, D. , Migaud, M. , Musi, N. , & Baur, J. A. (2023). Nicotinamide adenine dinucleotide in aging biology: Potential applications and many unknowns. Endocrine Reviews, 44, 1047–1073. - PubMed
-
- Bhatraju, P. K. , Ghassemieh, B. J. , Nichols, M. , Kim, R. , Jerome, K. R. , Nalla, A. K. , Greninger, A. L. , Pipavath, S. , Wurfel, M. M. , Evans, L. , Kritek, P. A. , West, T. E. , Luks, A. , Gerbino, A. , Dale, C. R. , Goldman, J. D. , O'Mahony, S. , & Mikacenic, C. (2020). Covid‐19 in critically ill patients in the Seattle region—case series. The New England Journal of Medicine, 382, 2012–2022. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

