Development and evaluation of an 18F-labeled nanobody to target SARS-CoV-2's spike protein
- PMID: 39354971
- PMCID: PMC11440877
- DOI: 10.3389/fnume.2022.1033697
Development and evaluation of an 18F-labeled nanobody to target SARS-CoV-2's spike protein
Abstract
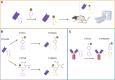
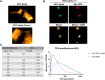
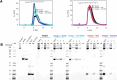
COVID-19, caused by the SARS-CoV-2 virus, has become a global pandemic that is still present after more than two years. COVID-19 is mainly known as a respiratory disease that can cause long-term consequences referred to as long COVID. Molecular imaging of SARS-CoV-2 in COVID-19 patients would be a powerful tool for studying the pathological mechanisms and viral load in different organs, providing insights into the disease and the origin of long-term consequences and assessing the effectiveness of potential COVID-19 treatments. Current diagnostic methods used in the clinic do not allow direct imaging of SARS-CoV-2. In this work, a nanobody (NB) - a small, engineered protein derived from alpacas - and an Fc-fused NB which selectively target the SARS-CoV-2 Spike protein were developed as imaging agents for positron emission tomography (PET). We used the tetrazine ligation to 18F-label the NB under mild conditions once the NBs were successfully modified with trans-cyclooctenes (TCOs). We confirmed binding to the Spike protein by SDS-PAGE. Dynamic PET scans in rats showed excretion through the liver for both constructs. Future work will evaluate in vivo binding to the Spike protein with our radioligands.
Keywords: COVID-19; PET; SARS-CoV-2; alpaca; biodistribution; nanobodies; tetrazine ligation.
© 2022 Lopes van den Broek, García-Vázquez, Andersen, Valenzuela-Nieto, Shalgunov, Battisti, Schwefel, Modhiran, Kramer, Cheuquemilla, Jara, Salinas-Varas, Amarilla, Watterson, Rojas-Fernandez and Herth.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- WHO. WHO Coronavirus (COVID-19) Dashbord (2022).
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

