Visual mismatch negativity in Parkinson's psychosis and potential for testing treatment mechanisms
- PMID: 39355002
- PMCID: PMC11443450
- DOI: 10.1093/braincomms/fcae291
Visual mismatch negativity in Parkinson's psychosis and potential for testing treatment mechanisms
Abstract
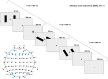
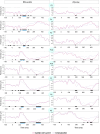
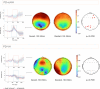
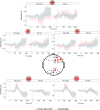
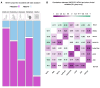
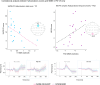
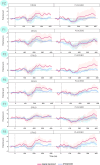
Psychosis and visual hallucinations are a prevalent non-motor symptom of Parkinson's disease, negatively affecting patients' quality of life and constituting a greater risk for dementia. Understanding neural mechanisms associated to these symptoms is instrumental for treatment development. The mismatch negativity is an event-related potential evoked by a violation in a sequence of sensory events. It is widely considered an index of sensory change-detection. Reduced mismatch negativity response is one of the most replicated results in schizophrenia and has been suggested to be a superior psychosis marker. To understand whether this event-related potential component could be a similarly robust marker for Parkinson's psychosis, we used electroencephalography with a change-detection task to study the mismatch negativity in the visual modality in 20 participants with Parkinson's and visual hallucinations and 18 matched Parkinson's participants without hallucinations. We find that visual mismatch negativity is clearly present in participants with Parkinson's disease without hallucinations at both parieto-occipital and frontal sites, whereas participants with Parkinson's and visual hallucinations show reduced or no differences in the two waveforms, confirming the sensitivity of mismatch negativity to psychosis, even within the same diagnostic group. We also explored the relationship between hallucination severity and visual mismatch negativity amplitude, finding a negative correlation between visual hallucinations severity scores and visual mismatch negativity amplitude at a central frontal and a parieto-occipital electrodes, whereby the more severe or complex (illusions, formed visual hallucinations) the symptoms the smaller the amplitude. We have also tested the potential role of the serotonergic 5-HT2A cascade in visual hallucinations in Parkinson's with these symptoms, following the receptor trafficking hypothesis. We did so with a pilot study in healthy controls (N = 18) providing support for the role of the Gi/o-dependent pathway in the psychedelic effect and a case series in participants with Parkinson's and visual hallucinations (N = 5) using a double-blind crossover design. Positive results on psychosis scores and mismatch amplitude add further to the potential role of serotonergic modulation of visual hallucinations in Parkinson's disease.
Keywords: 5-HT2A; Parkinson’s disease; Parkinson’s psychosis; mismatch negativity; visual hallucinations.
© The Author(s) 2024. Published by Oxford University Press on behalf of the Guarantors of Brain.
Conflict of interest statement
The authors do not have any competing interests to declare.
Figures


References
-
- Näätänen R, Kujala T, Kreegipuu K, et al. The mismatch negativity: An index of cognitive decline in neuropsychiatric and neurological diseases and in ageing. Brain. 2011;134:3435–3453. - PubMed
-
- Rao RP, Ballard DH. Predictive coding in the visual cortex: A functional interpretation of some extra-classical receptive-field effects. Nat Neurosci. 1999;2(1):79–87. - PubMed
LinkOut - more resources
Full Text Sources
