Investigating the Correlation Between Long-Term Response in Patients with Metastatic HER2+ Breast Cancer and the Activity of Regulatory T Cells: A Retrospective Study
- PMID: 39355199
- PMCID: PMC11444060
- DOI: 10.2147/BCTT.S470570
Investigating the Correlation Between Long-Term Response in Patients with Metastatic HER2+ Breast Cancer and the Activity of Regulatory T Cells: A Retrospective Study
Abstract
Background: Trastuzumab is commonly utilized in the management of metastatic HER2-positive breast cancer. Our main goal was to examine the clinical outcomes and immune markers of patients who received trastuzumab and chemotherapy treatment.
Methods: Between 1995 and 2012, a total of 98 patients diagnosed with metastatic HER2-positive breast cancer were retrospectively analyzed at Ege University Hospital and Tepecik Training and Research Hospital. The clinicopathological characteristics and clinical outcomes of the patients were assessed, and the associations between response rates, survival and the immune profiles of tumor infiltrating lymphocytes were statistically evaluated.
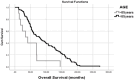
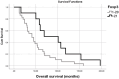
Results: The average age of patients at the time of diagnosis was 50.1±10.3 (ranging from 30 to 79) years. The mean follow-up period for all patients was 97.9±53.8 months. Among the patients, complete response was observed in 24.5%, partial response in 61.2%, and stable disease in 8.2% of cases. The average progression-free survival was 50.3±26.9 months (ranging from 1 to 163 months), and the average overall survival was 88.8±59.4 months (ranging from 12 to 272 months). After analyzing all cases, it was found that patients who were younger (p=0.006), exhibited higher CD3-positivity (p=0.041), presented with higher FOXP3-positivity (p=0.025), showed complete or at least partial response to treatment (p=0.008), and experienced a long-term response to trastuzumab (and chemotherapy) treatment had longer survival (p=0.001).
Conclusion: Patients with HER2-positive breast cancer, who initially respond positively to palliative trastuzumab and chemotherapy treatment, can achieve long-term tumor remission lasting for several years.
Keywords: forkhead box p3; long-term response; metastatic breast cancer; trastuzumab.
© 2024 Degirmenci et al.
Conflict of interest statement
The authors all affirm that they do not have any conflicts of interest.
Figures



References
-
- Cobleigh MA, Vogel CL, Tripathy D, et al. Multinational study of the efficacy and safety of humanized anti-HER2monoclonal antibody in women who have HER2-overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J Clin Oncol. 1999;17:2639–2648. doi: 10.1200/JCO.1999.17.9.2639 - DOI - PubMed
-
- Romond E, Suman VJ, Jeong J-H, et al. National Surgical Adjuvant Breast and Bowel Project (NSABP) Operations and Biostatistical Centers Trastuzumab plus adjuvant chemotherapy for HER2-positive breast cancer: final planned joint analysis of overall survival (OS) from NSABP B-31 and NCCTG N9831. Cancer Res. 2012;72(suppl 3):abstrS5–abstrS5.
-
- Papaldo P, Fabi A, Ferretti G, et al. A Phase II study on metastatic breast cancer patients treated with weekly vinorelbine with or without trastuzumab according to HER2 expression: changing the natural history of HER2-positive disease. Ann Oncol. 2006;17:630–636. doi: 10.1093/annonc/mdj110 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

