Phage therapy: A novel approach against multidrug-resistant pathogens
- PMID: 39355200
- PMCID: PMC11442959
- DOI: 10.1007/s13205-024-04101-8
Phage therapy: A novel approach against multidrug-resistant pathogens
Abstract
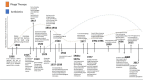
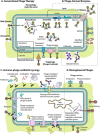
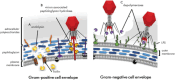
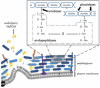
The rapid rise of multidrug-resistant (MDR) organisms has created a critical need for alternative treatment options. Phage therapy is gaining attention as an effective way to fight bacterial infections by using lytic bacteriophages to specifically target and kill harmful bacteria. This review discusses several phage therapeutic options and emphasizes new developments in phage biology. Phage treatment has proven to be successful against MDR bacteria, as evidenced by multiple human clinical trials that indicate favorable results in treating a range of diseases caused by these pathogens. Despite these promising results, challenges such as phage resistance, regulatory hurdles, and the need for standardized treatment protocols remain. To effectively combat MDR bacterial infections, future research must focus on enhancing phage effectiveness, guaranteeing safety for human usage and incorporating phage therapy into clinical practice.
Keywords: Antimicrobial resistance; Bacterial disease; Bacteriophage; Drug-resistant pathogens; Phage therapy.
© The Author(s) 2024.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources

