Advances and challenges in immunoPET methodology
- PMID: 39355220
- PMCID: PMC11440922
- DOI: 10.3389/fnume.2024.1360710
Advances and challenges in immunoPET methodology
Abstract
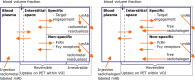
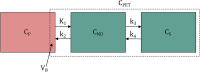
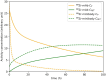
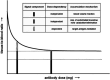
Immuno-positron emission tomography (immunoPET) enables imaging of specific targets that play a role in targeted therapy and immunotherapy, such as antigens on cell membranes, targets in the disease microenvironment, or immune cells. The most common immunoPET applications use a monoclonal antibody labeled with a relatively long-lived positron emitter such as 89Zr (T 1/2 = 78.4 h), but smaller antibody-based constructs labeled with various other positron emitting radionuclides are also being investigated. This molecular imaging technique can thus guide the development of new drugs and may have a pivotal role in selecting patients for a particular therapy. In early phase immunoPET trials, multiple imaging time points are used to examine the time-dependent biodistribution and to determine the optimal imaging time point, which may be several days after tracer injection due to the slow kinetics of larger molecules. Once this has been established, usually only one static scan is performed and semi-quantitative values are reported. However, total PET uptake of a tracer is the sum of specific and nonspecific uptake. In addition, uptake may be affected by other factors such as perfusion, pre-/co-administration of the unlabeled molecule, and the treatment schedule. This article reviews imaging methodologies used in immunoPET studies and is divided into two parts. The first part summarizes the vast majority of clinical immunoPET studies applying semi-quantitative methodologies. The second part focuses on a handful of studies applying pharmacokinetic models and includes preclinical and simulation studies. Finally, the potential and challenges of immunoPET quantification methodologies are discussed within the context of the recent technological advancements provided by long axial field of view PET/CT scanners.
Keywords: PET/CT; imaging; immunoPET; kinetic modeling; monoclonal antibody; quantification; zirconium.
© 2024 Mohr, van Sluis, Lub-de Hooge, Lammertsma, Brouwers and Tsoumpas.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures
Similar articles
-
Quantitative immunoPET of prostate cancer xenografts with 89Zr- and 124I-labeled anti-PSCA A11 minibody.J Nucl Med. 2014 Mar;55(3):452-9. doi: 10.2967/jnumed.113.120873. Epub 2014 Feb 6. J Nucl Med. 2014. PMID: 24504052 Free PMC article.
-
Development of a novel long-lived immunoPET tracer for monitoring lymphoma therapy in a humanized transgenic mouse model.Bioconjug Chem. 2012 Jun 20;23(6):1221-9. doi: 10.1021/bc300039r. Epub 2012 Jun 11. Bioconjug Chem. 2012. PMID: 22621257 Free PMC article.
-
Preclinical ImmunoPET Imaging of Glioblastoma-Infiltrating Myeloid Cells Using Zirconium-89 Labeled Anti-CD11b Antibody.Mol Imaging Biol. 2020 Jun;22(3):685-694. doi: 10.1007/s11307-019-01427-1. Mol Imaging Biol. 2020. PMID: 31529407 Free PMC article.
-
Copper-64-immunoPET imaging: bench to bedside.Q J Nucl Med Mol Imaging. 2020 Dec;64(4):356-363. doi: 10.23736/S1824-4785.20.03310-5. Epub 2020 Oct 12. Q J Nucl Med Mol Imaging. 2020. PMID: 33045821 Review.
-
Zirconium 89 and Copper 64 for ImmunoPET: From Antibody Bioconjugation and Radiolabeling to Molecular Imaging.Pharmaceutics. 2024 Jun 30;16(7):882. doi: 10.3390/pharmaceutics16070882. Pharmaceutics. 2024. PMID: 39065579 Free PMC article. Review.
Cited by
-
Ultra-low dose immunoPET using 64Cu-rituximab tracer for a human CD20 mouse model.Front Med (Lausanne). 2025 Apr 7;12:1548132. doi: 10.3389/fmed.2025.1548132. eCollection 2025. Front Med (Lausanne). 2025. PMID: 40259986 Free PMC article.
-
Synthesis and In Vitro Evaluation of a Scandium-44 Radiolabeled Nanobody as a PD-L1 PET Imaging Probe.Pharmaceutics. 2025 Jun 19;17(6):796. doi: 10.3390/pharmaceutics17060796. Pharmaceutics. 2025. PMID: 40574107 Free PMC article.
-
Advancements in non-invasive visualization of the immune checkpoint TIGIT: a systematic review.Eur J Nucl Med Mol Imaging. 2025 Jul 15. doi: 10.1007/s00259-025-07439-0. Online ahead of print. Eur J Nucl Med Mol Imaging. 2025. PMID: 40663162 Review.
-
Good practices for 89Zr radiopharmaceutical production and quality control.EJNMMI Radiopharm Chem. 2024 May 11;9(1):40. doi: 10.1186/s41181-024-00258-y. EJNMMI Radiopharm Chem. 2024. PMID: 38733556 Free PMC article. Review.
References
-
- Van Dongen GA, Huisman MC, Boellaard R, Harry Hendrikse N, Windhorst AD, Visser GW, et al. 89Zr-immuno-PET For imaging of long circulating drugs and disease targets: why, how and when to be applied? Q J Nucl Med Mol Imaging. (2015) 59(1):18–38. https://www.minervamedica.it/en/journals/nuclear-med-molecular-imaging/a... - PubMed
-
- Jauw YWS, Menke-van der Houven van Oordt CW, Hoekstra OS, Hendrikse NH, Vugts DJ, Zijlstra JM, et al. Immuno-positron emission tomography with zirconium-89-labeled monoclonal antibodies in oncology: what can we learn from initial clinical trials? Front Pharmacol. (2016) 7:131. 10.3389/fphar.2016.00131 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

