Tether-entangled conjugated helices
- PMID: 39355229
- PMCID: PMC11440437
- DOI: 10.1039/d4sc04796f
Tether-entangled conjugated helices
Abstract
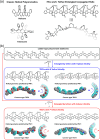
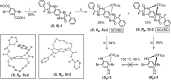
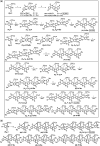
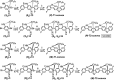
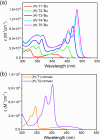
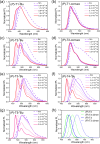
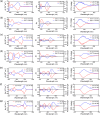
A new design concept, tether-entangled conjugated helices (TECHs), is introduced for helical polyaromatic molecules. TECHs consist of a linear polyaromatic ladder backbone and periodically entangling tethers with the same planar chirality. By limiting the length of tether, all tethers synchronously bend and twist the backbone with the same manner, and change it into a helical ribbon with a determinate helical chirality. The 3D helical features are customizable via modular synthesis by using two types of synthons, the planar chiral tethering unit (C 2 symmetry) and the docking unit (C 2h symmetry), and no post chiral resolution is needed. Moreover, TECHs possess persistent chiral properties due to the covalent locking of helical configuration by tethers. Concave-type and convex-type oligomeric TECHs are prepared as a proof-of-concept. Unconventional double-helix π-dimers are observed in the single crystals of concave-type TECHs. Theoretical studies indicate the smaller binding energies in double-helix π-dimers than conventional planar π-dimers. A concentration-depend emission is found for concave-type TECHs, probably due to the formation of double-helix π-dimers in the excited state. All TECHs show strong circularly polarized luminescence (CPL) with dissymmetric factors (|g lum|) generally over 10-3. Among them, the (P)-T4-tBu shows the highest |g lum| of 1.0 × 10-2 and a high CPL brightness of 316 M-1 cm-1.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Shen Y. Chen C. F. Helicenes: synthesis and applications. Chem. Rev. 2012;112:1463–1535. - PubMed
-
- Zhang D. W. Li M. Chen C. F. Recent advances in circularly polarized electroluminescence based on organic light-emitting diodes. Chem. Soc. Rev. 2020;49:1331–1343. - PubMed
-
- Anderson H. V. Gois N. D. Chalifoux W. A. New advances in chiral nanographene chemistry. Org. Chem. Front. 2023;10:4167–4197.
-
- Zhu Y. Wang J. Helical Synthetic Nanographenes with Atomic Precision. Acc. Chem. Res. 2023;56:363–373. - PubMed
-
- Yang Y. da Costa R. C. Fuchter M. J. Campbell A. J. Circularly polarized light detection by a chiral organic semiconductor transistor. Nat. Photonics. 2013;7:634–638.
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

