The iNKT cell ligand α-GalCer prevents murine septic shock by inducing IL10-producing iNKT and B cells
- PMID: 39355237
- PMCID: PMC11442275
- DOI: 10.3389/fimmu.2024.1457690
The iNKT cell ligand α-GalCer prevents murine septic shock by inducing IL10-producing iNKT and B cells
Abstract
Introduction: α-galactosylceramide (α-GalCer), a prototypical agonist of invariant natural killer T (iNKT) cells, stimulates iNKT cells to produce various cytokines such as IFNγ and IL4. Moreover, repeated α-GalCer treatment can cause protective or pathogenic outcomes in various immune-mediated diseases. However, the precise role of α-GalCer-activated iNKT cells in sepsis development remains unclear. To address this issue, we employed a lipopolysaccharide (LPS)/D-galactosamine (D-GalN)-induced murine sepsis model and two alternative models.
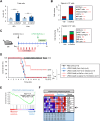
Methods: Sepsis was induced in wild-type (WT) C57BL/6 (B6) mice by three methods (LPS/D-GalN, α-GalCer/D-GalN, and cecal slurry), and these mice were monitored for survival rates. WT B6 mice were intraperitoneally injected with α-GalCer or OCH (an IL4-biased α-GalCer analog) one week prior to the induction of sepsis. To investigate the effects of α-GalCer-mediated iNKT cell activation on sepsis development, immune responses were analyzed by flow cytometry using splenocytes and liver-infiltrating leukocytes. In addition, a STAT6 inhibitor (AS1517499) and an IL10 inhibitor (AS101) were employed to evaluate the involvement of IL4 or IL10 signaling. Furthermore, we performed B cell adoptive transfers to examine the contribution of α-GalCer-induced regulatory B (Breg) cell populations in sepsis protection.
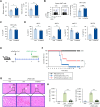
Results: In vivo α-GalCer pretreatment polarized iNKT cells towards IL4- and IL10-producing phenotypes, significantly attenuating LPS/D-GalN-induced septic lethality in WT B6 mice. Furthermore, α-GalCer pretreatment reduced the infiltration of immune cells to the liver and attenuated pro-inflammatory cytokine production. Treatment with a STAT6 inhibitor was unable to modulate disease progression, indicating that IL4 signaling did not significantly affect iNKT cell-mediated protection against sepsis. This finding was confirmed by pretreatment with OCH, which did not alter sepsis outcomes. However, interestingly, prophylactic effects of α-GalCer on sepsis were significantly suppressed by treatment with an IL10 antagonist, suggesting induction of IL10-dependent anti-inflammatory responses. In addition to IL10-producing iNKT cells, IL10-producing B cell populations were significantly increased after α-GalCer pretreatment.
Conclusion: Overall, our results identify α-GalCer-mediated induction of IL10 by iNKT and B cells as a promising option for controlling the pathogenesis of postoperative sepsis.
Keywords: B cells; IL10; iNKT cells; sepsis; α-GalCer.
Copyright © 2024 Park, Lee, Kim, Park, Van Kaer and Hong.
Conflict of interest statement
LVK is a scientific advisory board member of Isu Abxis Co., Ltd. Republic of Korea. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

