Robust and persistent B-cell responses following SARS-CoV-2 vaccine determine protection from SARS-CoV-2 infection
- PMID: 39355249
- PMCID: PMC11442242
- DOI: 10.3389/fimmu.2024.1445653
Robust and persistent B-cell responses following SARS-CoV-2 vaccine determine protection from SARS-CoV-2 infection
Abstract
Introduction: A clear immune correlate of protection from severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection has not been defined. We explored antibody, B-cell, and T-cell responses to the third-dose vaccine and relationship to incident SARS-CoV-2 infection.
Methods: Adults in a prospective cohort provided blood samples at day 0, day 14, and 10 months after the third-dose SARS-CoV-2 vaccine. Participants self-reported incident SARS-CoV-2 infection. Plasma anti-SARS-CoV-2 receptor-binding domain (RBD) and spike-subunit-1 and spike-subunit-2 antibodies were measured. A sub-study assessed SARS-CoV-2-specific plasma and memory B-cell and memory T-cell responses in peripheral blood mononuclear cells by enzyme-linked immunospot. Comparative analysis between participants who developed incident infection and uninfected participants utilised non-parametric t-tests, Kaplan-Meier survival analysis, and Cox proportional hazard ratios.
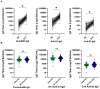
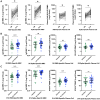
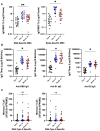
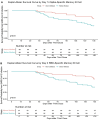
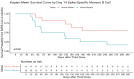
Results: Of the 132 participants, 47 (36%) reported incident SARS-CoV-2 infection at a median 16.5 (16.25-21) weeks after the third-dose vaccination. RBD titres and B-cell responses, but not T-cell responses, increased after the third-dose vaccine. Whereas no significant difference in day 14 antibody titres or T-cell responses was observed between participants with and without incident SARS-CoV-2 infection, RBD memory B-cell frequencies were significantly higher in those who did not develop infection [10.0% (4.5%-16.0%) versus 4.9% (1.6%-9.3%), p = 0.01]. RBD titres and memory B-cell frequencies remained significantly higher at 10 months than day 0 levels (p < 0.01).
Discussion: Robust antibody and B-cell responses persisted at 10 months following the third-dose vaccination. Higher memory B-cell frequencies, rather than antibody titres or T-cell responses, predicted protection from subsequent infection, identifying memory B cells as a correlate of protection.
Keywords: B cells; COVID-19; COVID-19 vaccine; SARS-CoV-2; T cells; immunogenicity.
Copyright © 2024 Byrne, Gu, Garcia-Leon, Gaillard, Saini, Alalwan, Tomás-Cortázar, Kenny, Donohue, Reynolds, O’Gorman, Landay, Doran, Stemler, Koehler, Cox, Olesen, Lelievre, O’Broin, Savinelli, Feeney, O’Halloran, Cotter, Horgan, Kelly, Sadlier, de Barra, Cornely, Gautier, Mallon and All Ireland Infectious Diseases cohort study and VACCELERATE consortium.
Conflict of interest statement
JS has received research support by the German Ministry of Education and Research BMBF, Basilea Pharmaceuticals, Noscendo; has received speaker honoraria by AbbVie, Gilead, Hikma and Pfizer; has been a consultant to Gilead, Alvea Vax. and Micron Research PK reports grants or contracts from German Federal Ministry of Research and Education BMBF B-FAST Bundesweites Forschungsnetz Angewandte Surveillance und Testung and NAPKON Nationales Pandemie Kohorten Netz, German National Pandemic Cohort Network of the Network University Medicine NUM and the State of North Rhine-Westphalia; Consulting fees Ambu GmbH, Gilead Sciences, infill healthcare communication GmbH, Mundipharma Resarch Limited, Noxxon N.V. and Pfizer Pharma; Honoraria for lectures from Akademie für Infektionsmedizin e.V., Ambu GmbH, Astellas Pharma, BioRad Laboratories Inc., Datamed GmbH, European Confederation of Medical Mycology, Gilead Sciences, GPR Academy Ruesselsheim, HELIOS Kliniken GmbH, Jazz Pharmaceuticals Germany GmbH, Lahn-Dill-Kliniken GmbH, medupdate GmbH, MedMedia GmbH, MSD Sharp & Dohme GmbH, Pfizer Pharma GmbH, Scilink Comunicación Científica SC, streamedup! GmbH, University Hospital and LMU Munich and VITIS GmbH; Participation on an Advisory Board from Ambu GmbH, Gilead Sciences, Mundipharma Resarch Limited and Pfizer Pharma; A filed patent at the German Patent and Trade Mark Office DE 10 2021 113 007.7; Other non-financial interests from Elsevier, Wiley and Taylor & Francis online outside the submitted work. SS has received financial support by Gilead Sciences, ViiV Healthcare, and MSD for attendance to international conferences. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Doherty J, O'Morain N, Stack R, Tosetto M, Inzitiari R, O'Reilly S, et al. . Reduced serological response to COVID-19 booster vaccine is associated with reduced B cell memory in patients with inflammatory bowel disease; VARIATION [VAriability in response in IBD againsT SARS-COV-2 immunisatiON. J Crohns Colitis. (2023) 17:1445–56. doi: 10.1093/ecco-jcc/jjad065 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

