Tonsil explants as a human in vitro model to study vaccine responses
- PMID: 39355250
- PMCID: PMC11442277
- DOI: 10.3389/fimmu.2024.1425455
Tonsil explants as a human in vitro model to study vaccine responses
Abstract
Introduction: Vaccination is one of the most effective infection prevention strategies. Viruses with high mutation rates -such as influenza- escape vaccine-induced immunity and represent significant challenges to vaccine design. Influenza vaccine strain selection is based on circulating strains and immunogenicity testing in animal models with limited predictive outcomes for vaccine effectiveness in humans.
Methods: We developed a human in vitro vaccination model using human tonsil tissue explants cultured in 3D perfusion bioreactors to be utilized as a platform to test and improve vaccines.
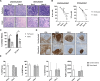
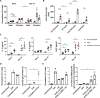
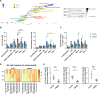
Results: Tonsils cultured in bioreactors showed higher viability, metabolic activity, and more robust immune responses than those in static cultures. The in vitro vaccination system responded to various premanufactured vaccines, protein antigens, and antigen combinations. In particular, a multivalent in vitro immunization with three phylogenetically distant H3N2 influenza strains showed evidence for broader B cell activation and induced higher antibody cross-reactivity than combinations with more related strains. Moreover, we demonstrate the capacity of our in vitro model to generate de novo humoral immune responses to a model antigen.
Discussion: Perfusion-cultured tonsil tissue may be a valuable human in vitro model for immunology research with potential application in vaccine candidate selection.
Keywords: antibodies; bioreactor; human; in vitro model; influenza; tonsil; vaccines.
Copyright © 2024 Bonaiti, Muraro, Robert, Jakscha, Dirnhofer, Martin and Berger.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision
Figures



References
-
- Messina NL, Germano S, McElroy R, Bonnici R, Grubor-Bauk B, Lynn DJ, et al. . Specific and off-target immune responses following COVID-19 vaccination with ChAdOx1-S and BNT162b2 vaccines-an exploratory sub-study of the BRACE trial. EBioMedicine. (2024) 103:105100. doi: 10.1016/j.ebiom.2024.105100 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

