A Retrospective Assessment of Guideline Adherence and Treatment Outcomes From Clostridioides difficile Infection Following the IDSA 2021 Clinical Guideline Update: Clostridioides difficile Infection
- PMID: 39355263
- PMCID: PMC11443340
- DOI: 10.1093/ofid/ofae524
A Retrospective Assessment of Guideline Adherence and Treatment Outcomes From Clostridioides difficile Infection Following the IDSA 2021 Clinical Guideline Update: Clostridioides difficile Infection
Abstract
Background: The 2021 update to the Infectious Diseases Society of America Clostridioides difficile infection (CDI) guidelines recommended fidaxomicin as the preferred treatment over vancomycin for patients with initial and recurrent CDI. Few studies have examined how treatment patterns and clinical outcomes of hospitalized CDI patients changed after the postguideline update or contemporary real-world outcomes of fidaxomicin vs vancomycin.
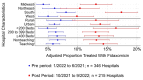
Methods: This retrospective, observational study used the PINC AI Healthcare Database on adult patients who received CDI treatment between 1/2020 and 6/2021 (pre period) and between 10/2021 and 9/2022 (post period). We examined treatment patterns of fidaxomicin, vancomycin, and metronidazole, as well as clinical and health care resource use outcomes of patients treated exclusively with fidaxomicin vs vancomycin, using nearest-neighbor propensity matching and hierarchical regression methods. As a sensitivity analysis, we repeated the fidaxomicin vs vancomycin comparisons among patients with recurrent and nonrecurrent index infections.
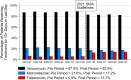
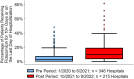
Results: A total of 45 049 patients with CDI from 779 US hospitals met initial inclusion criteria. Comparing the pre vs post periods, the proportion of patients treated with fidaxomicin increased from 5.9% to 13.7% (P < .001), vancomycin use decreased from 87.9% to 82.9% (P < .001), and metronidazole use decreased from 21.6% to 17.2% (P < .001). When comparing fidaxomicin vs vancomycin in the post period, fidaxomicin was associated with lower CDI recurrence (6.1% vs 10.2%; P < .001) and higher sustained clinical response (91.7% vs 87.8%; P < .001). Ninety-day postdischarge costs were not significantly different between groups. A sensitivity analyses showed similar findings.
Conclusions: Since the 2021 guideline update, fidaxomicin use has increased significantly but could be further utilized given its association with better clinical outcomes and no increase in postdischarge costs.
Keywords: 2021 clinical guideline update; Clostridioides difficile; fidaxomicin; metronidazole; vancomycin.
© The Author(s) 2024. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. E.R.D. has received relevant research support through grants from Theriva Biologics, Ferring, Pfizer, and AstraZeneca. E.R.D. has also received consulting fees from Merck & Co., Inc. (Rahway, NJ, USA), Seres, Ferring, AstraZeneca, Abbott, Pfizer, Summit, GSK, and Recursion. Q.L. was an employee of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc. (Rahway, NJ, USA), at the time this study was conducted but is currently a full-time employee of Takeda. E.N.O., V.T., and F.S. are employees of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc. (Rahway, NJ, USA). B.H.N.'s company, OptiStatim, LLC, has received consulting fees from Merck & Co., Inc. (Rahway, NJ, USA).
Figures
References
-
- McDonald LC, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis 2018; 66:e1–48. - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

