Inhibition of cytotoxic self-assembly of HEWL through promoting fibrillation by new synthesized α-hydroxycarbamoylphosphinic acids
- PMID: 39355328
- PMCID: PMC11443501
- DOI: 10.1039/d4ra02969k
Inhibition of cytotoxic self-assembly of HEWL through promoting fibrillation by new synthesized α-hydroxycarbamoylphosphinic acids
Abstract
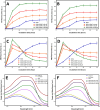
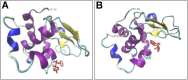
The main objective of the present study is to investigate the potency of new synthesized hydroxycarbamoyl phosphinic acid derivatives in modulating cytotoxic fibrillogenesis of hen egg white lysozyme (HEWL), as a common model in protein aggregation studies. Hydroxycarbamoyl phosphinic acid derivatives were prepared by the reaction of α-hydroxyalkylphosphinic acids with isocyanates (or isothiocyanates) in the presence of trimethylsilyl chloride (TMSCl). The designed process involves the condensation reaction leading to formation of new C sp2-P bond formation. The synthesis and purity of novel designed compounds were confirmed by NMR, LC-MS, and HPLC techniques. A range of experiments, including thioflavin T (ThT) and 8-anilino-1-naphthalenesulfonic acid (ANS) fluorescence assays, Congo red binding measurement, atomic force microscopy imaging, MTT-based cell viability and hemolysis assays were employed to investigate anti-amyloidogenic effects of tested compounds. The obtained results demonstrate that these compounds are able to significantly modulate the self-assembly process of HEWL via shortening of nucleation phase leading to the acceleration of fibrillation and appearance of very large and thick fibrils with decreased surface hydrophobicity and cytotoxicity. Based on ANS binding data, we suggest that increased exposure of hydrophobic patches of oligomeric species is the possible mechanism by which tested compounds promote self-assembly process of HEWL. Fluorescence anisotropy and molecular docking studies indicate the interaction of both synthesized compounds with HEWL, and more specifically with residues that are situated in the highly aggregation-prone β-domain region of protein. This study unveils the potential of hydroxyalkylphosphinic acids as modulators of amyloid fibrillation highlighting these compounds as a promising approach for targeting protein aggregates associated with neurodegenerative diseases.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures









References
LinkOut - more resources
Full Text Sources

