Predictors of the efficacy of vedolizumab in patients with ulcerative colitis
- PMID: 39355361
- PMCID: PMC11439607
- DOI: 10.18999/nagjms.86.3.407
Predictors of the efficacy of vedolizumab in patients with ulcerative colitis
Abstract
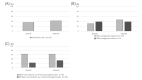
Vedolizumab is a treatment option for ulcerative colitis but data on predictors of treatment response remain insufficient to establish personalized treatment strategies. We aimed to investigate the real-world effectiveness of vedolizumab in adult patients with ulcerative colitis and explore factors involved in predicting treatment response. This single-center, single-arm, prospective observational study included 26 patients with clinically active ulcerative colitis patients' characteristics at baseline, epidemiological information, existing treatment, clinical activity index score, endoscopic score, and blood test data were collected. Serum levels of tumor necrosis factors alpha, interferon gamma, interleukin-4, interleukin-6, interleukin-10, interleukin-17, soluble mucosal addressin cell adhesion molecule 1, and soluble vascular cell adhesion molecule 1 were measured. Patient characteristics in the remission and non-remission groups were compared based on these parameters. Clinical remission at 6 weeks of treatment occurred in 9 (35%) of the 26 patients. At 14 weeks, clinical remission was observed in 11 patients (42%). There were no significant differences pertaining to age, sex, duration of disease, extent of disease, steroid resistance, or prior treatment with biological agents among the two groups after 14 weeks of treatment. Hemoglobin ≥ 11.5 g/dL (odds ratio, 15.0; 95% confidence interval, 1.50-149; P=0.014) and soluble mucosal addressin cell adhesion molecule 1 ≥ 765 pg/mL (odds ratio, 17.3; 95% confidence interval, 2.36-127; P=0.004) were significant factors. In conclusion, hemoglobin and serum soluble mucosal addressin cell adhesion molecule 1 levels are factors correlated with the therapeutic efficacy of vedolizumab.
Keywords: MAdCAM-1; hemoglobin; predictive factor; ulcerative colitis; vedolizumab.
Conflict of interest statement
The authors received no funding for this study. The authors have no conflicts of interest to declare.
Figures



References
-
- Turner D, Ricciuto A, Lewis A, et al. STRIDE-II: an update on the selecting therapeutic targets in inflammatory bowel disease (STRIDE) Initiative of the International Organization for the Study of IBD (IOIBD): determining therapeutic goals for treat-to-target strategies in IBD. Gastroenterology. 2021;160(5):1570–1583. doi: 10.1053/j.gastro.2020.12.031. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
