Crucial roles of exosomes secreted from ganglioside GD3/GD2-positive glioma cells in enhancement of the malignant phenotypes and signals of GD3/GD2-negative glioma cells
- PMID: 39355364
- PMCID: PMC11439612
- DOI: 10.18999/nagjms.86.3.435
Crucial roles of exosomes secreted from ganglioside GD3/GD2-positive glioma cells in enhancement of the malignant phenotypes and signals of GD3/GD2-negative glioma cells
Abstract
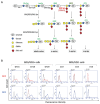
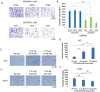
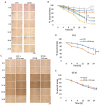
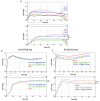
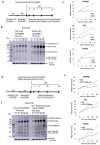
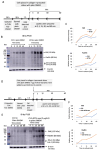
Neuroectoderm-derived tumors characteristically express gangliosides such as GD3 and GD2. Many studies have reported that gangliosides GD3/GD2 enhance malignant phenotypes of cancers. Recently, we reported that human gliomas expressing GD3/GD2 exhibited enhanced malignant phenotypes. Here, we investigated the function of GD3/GD2 in glioma cells and GD3/GD2-expressing glioma-derived exosomes. As reported previously, transfectant cells of human glioma U251 MG expressing GD3/GD2 showed enhanced cancer phenotypes compared with GD3/GD2-negative controls. When GD3/GD2-negative cells were treated with exosomes secreted from GD3/GD2-positive cells, clearly increased malignant properties were observed. Furthermore, increased phosphorylation of signaling molecules was detected after 5-15 min of exosome treatment, ie, higher tyrosine phosphorylation of platelet-derived growth factor receptor, focal adhesion kinase, and paxillin was found in treated cells than in controls. Phosphorylation of extracellular signal-regulated kinase-1/2 was also enhanced. Consequently, it is suggested that exosomes secreted from GD3/GD2-positive gliomas play important roles in enhancement of the malignant properties of glioma cells, leading to total aggravation of heterogenous cancer tissues, and also in the regulation of tumor microenvironments.
Keywords: exosome; ganglioside; glioma; malignancy.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


Similar articles
-
Action Mechanisms of Exosomes Derived from GD3/GD2-Positive Glioma Cells in the Regulation of Phenotypes and Intracellular Signaling: Roles of Integrins.Int J Mol Sci. 2024 Nov 27;25(23):12752. doi: 10.3390/ijms252312752. Int J Mol Sci. 2024. PMID: 39684463 Free PMC article.
-
Enhancement of malignant properties of human glioma cells by ganglioside GD3/GD2.Int J Oncol. 2018 Apr;52(4):1255-1266. doi: 10.3892/ijo.2018.4266. Epub 2018 Feb 7. Int J Oncol. 2018. PMID: 29436609
-
Enhancement of malignant properties of human osteosarcoma cells with disialyl gangliosides GD2/GD3.Cancer Sci. 2012 Sep;103(9):1656-64. doi: 10.1111/j.1349-7006.2012.02344.x. Epub 2012 Jul 16. Cancer Sci. 2012. PMID: 22632091 Free PMC article.
-
Disialyl gangliosides enhance tumor phenotypes with differential modalities.Glycoconj J. 2012 Dec;29(8-9):579-84. doi: 10.1007/s10719-012-9423-0. Epub 2012 Jul 5. Glycoconj J. 2012. PMID: 22763744 Review.
-
GD2 is a Crucial Ganglioside in the Signal Modulation and Application as a Target of Cancer Therapeutics.Cancer Sci. 2025 Apr;116(4):862-870. doi: 10.1111/cas.70011. Epub 2025 Feb 7. Cancer Sci. 2025. PMID: 39921214 Free PMC article. Review.
Cited by
-
Action Mechanisms of Exosomes Derived from GD3/GD2-Positive Glioma Cells in the Regulation of Phenotypes and Intracellular Signaling: Roles of Integrins.Int J Mol Sci. 2024 Nov 27;25(23):12752. doi: 10.3390/ijms252312752. Int J Mol Sci. 2024. PMID: 39684463 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
