The mesolimbic system and the loss of higher order network features in schizophrenia when learning without reward
- PMID: 39355381
- PMCID: PMC11443173
- DOI: 10.3389/fpsyt.2024.1337882
The mesolimbic system and the loss of higher order network features in schizophrenia when learning without reward
Abstract
Introduction: Schizophrenia is characterized by a loss of network features between cognition and reward sub-circuits (notably involving the mesolimbic system), and this loss may explain deficits in learning and cognition. Learning in schizophrenia has typically been studied with tasks that include reward related contingencies, but recent theoretical models have argued that a loss of network features should be seen even when learning without reward. We tested this model using a learning paradigm that required participants to learn without reward or feedback. We used a novel method for capturing higher order network features, to demonstrate that the mesolimbic system is heavily implicated in the loss of network features in schizophrenia, even when learning without reward.
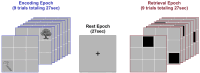
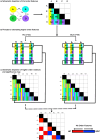
Methods: fMRI data (Siemens Verio 3T) were acquired in a group of schizophrenia patients and controls (n=78; 46 SCZ, 18 ≤ Age ≤ 50) while participants engaged in associative learning without reward-related contingencies. The task was divided into task-active conditions for encoding (of associations) and cued-retrieval (where the cue was to be used to retrieve the associated memoranda). No feedback was provided during retrieval. From the fMRI time series data, network features were defined as follows: First, for each condition of the task, we estimated 2nd order undirected functional connectivity for each participant (uFC, based on zero lag correlations between all pairs of regions). These conventional 2nd order features represent the task/condition evoked synchronization of activity between pairs of brain regions. Next, in each of the patient and control groups, the statistical relationship between all possible pairs of 2nd order features were computed. These higher order features represent the consistency between all possible pairs of 2nd order features in that group and embed within them the contributions of individual regions to such group structure.
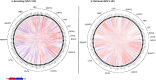
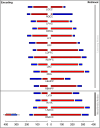
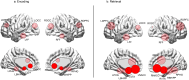
Results: From the identified inter-group differences (SCZ ≠ HC) in higher order features, we quantified the respective contributions of individual brain regions. Two principal effects emerged: 1) SCZ were characterized by a massive loss of higher order features during multiple task conditions (encoding and retrieval of associations). 2) Nodes in the mesolimbic system were over-represented in the loss of higher order features in SCZ, and notably so during retrieval.
Discussion: Our analytical goals were linked to a recent circuit-based integrative model which argued that synergy between learning and reward circuits is lost in schizophrenia. The model's notable prediction was that such a loss would be observed even when patients learned without reward. Our results provide substantial support for these predictions where we observed a loss of network features between the brain's sub-circuits for a) learning (including the hippocampus and prefrontal cortex) and b) reward processing (specifically constituents of the mesolimbic system that included the ventral tegmental area and the nucleus accumbens. Our findings motivate a renewed appraisal of the relationship between reward and cognition in schizophrenia and we discuss their relevance for putative behavioral interventions.
Keywords: cognition; functional MRI; learning; memory; mesolimbic system; schizophrenia.
Copyright © 2024 Martin, Chowdury, Kopchick, Thomas, Khatib, Rajan, Zajac-Benitez, Haddad, Amirsadri, Robison, Thakkar, Stanley and Diwadkar.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures

References
-
- Waltz JA, Xu Z, Brown EC, Ruiz RR, Frank MJ, Gold JM. Motivational deficits in schizophrenia are associated with reduced differentiation between gain and loss-avoidance feedback in the striatum. Biol Psychiatry Cogn Neurosci Neuroimaging. (2018) 3:239–47. doi: 10.1016/j.bpsc.2017.07.008 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

