Pilot Study of IL-1 Antagonist Anakinra for Treatment of Endometriosis
- PMID: 39355382
- PMCID: PMC11444056
- DOI: 10.2147/IJWH.S467041
Pilot Study of IL-1 Antagonist Anakinra for Treatment of Endometriosis
Abstract
Purpose: To evaluate the impact of an interleukin-1 (IL-1) antagonist anakinra (Kineret®) on endometriosis-related quality of life (QoL), pain, and inflammatory biomarkers.
Methods: This was a single-site, randomized, double-blinded, placebo-controlled, cross-over pilot clinical study of patients recruited at an academic specialty clinic. Eligible participants were females aged 18-45 years with menstrual cycles every 24-32 days. Subjects had moderate to severe dysmenorrhea and either a surgical diagnosis of endometriosis or an endometrioma on imaging. Subjects were randomly assigned in a double-blind fashion to receive either the study drug or placebo administered as daily injections during the first 3 periods and then the alternate intervention for the next 3 periods.
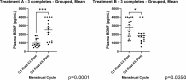
Results: Fifteen subjects completed the 6 menstrual cycle study. After each period, they completed the Endometriosis Health Profile-30 (EHP-30) QoL questionnaire and an assessment of dysmenorrhea using a 0-100 Visual Analogue Scale (VAS). All domains of the EHP-30 showed a trend towards improvement, with significant improvements in powerlessness (54.5 vs 63.3, p = 0.04) and self-image (58.1 vs 66.7, p = 0.03) on the study drug compared to placebo. The mean dysmenorrhea VAS also trended toward improvement with a score of 37.5 during active treatment and 42.6 with placebo (p = 0.26). No difference in menstrual cycle length was detected (29.3 days vs 27.7 days, p = 0.56). There were significant differences in multiple inflammatory biomarkers between the study drug and placebo, including BDNF, IL-1, and IL-6 among certain groups.
Conclusion: With all EHP-30 domains and the dysmenorrhea VAS showing either a statistical improvement or trend towards improvement, there is justification for a larger study. As no impact on menstrual cycles was detected, anakinra may be a particularly impactful option for women desiring fertility. Additional evaluation is needed on the role of anakinra on inflammatory markers given significant reductions were identified in multiple biomarkers.
Keywords: IL-1 antagonist; Kineret; anakinra; endometriosis; inflammation; pelvic pain; quality of life.
Plain language summary
Endometriosis is a common gynecologic disease afflicting millions of patients. Anakinra is an IL-1 antagonist currently used for treatment of rheumatoid arthritis which has been found to improve quality of life measures for patients with endometriosis. Anakinra also reduces levels of biomarkers known to be associated with endometriosis-related inflammation. More study is needed on the role of anakinra in improving endometriosis symptoms.
© 2024 Sullender et al.
Conflict of interest statement
Dr Foster and Dr Wessels are Co-Founders of Afynia Laboratories, a medical diagnostic company, and hold several patents unrelated to the treatment of endometriosis. The other authors report no conflicts of interest in this work. An abstract of this paper was presented at the 2023 AAGL Conference as a poster with interim findings. The poster’s abstract was published in the Journal of Minimally Invasive Gynecology Supplement: https://www.sciencedirect.com/science/article/pii/S1553465023006738
Figures


Similar articles
-
Once daily oral relugolix combination therapy versus placebo in patients with endometriosis-associated pain: two replicate phase 3, randomised, double-blind, studies (SPIRIT 1 and 2).Lancet. 2022 Jun 18;399(10343):2267-2279. doi: 10.1016/S0140-6736(22)00622-5. Lancet. 2022. PMID: 35717987 Clinical Trial.
-
Achieving clinically meaningful response in endometriosis pain symptoms is associated with improvements in health-related quality of life and work productivity: analysis of 2 phase III clinical trials.Am J Obstet Gynecol. 2020 Jun;222(6):592.e1-592.e10. doi: 10.1016/j.ajog.2019.11.1255. Epub 2019 Nov 22. Am J Obstet Gynecol. 2020. PMID: 31759891 Clinical Trial.
-
Impact of relugolix combination therapy on functioning and quality of life in women with endometriosis-associated pain.Fertil Steril. 2024 Oct;122(4):687-695. doi: 10.1016/j.fertnstert.2024.06.009. Epub 2024 Jun 19. Fertil Steril. 2024. PMID: 38906210 Clinical Trial.
-
Interventions for reducing inflammation in familial Mediterranean fever.Cochrane Database Syst Rev. 2018 Oct 19;10(10):CD010893. doi: 10.1002/14651858.CD010893.pub3. Cochrane Database Syst Rev. 2018. Update in: Cochrane Database Syst Rev. 2022 Mar 29;3:CD010893. doi: 10.1002/14651858.CD010893.pub4. PMID: 30338514 Free PMC article. Updated.
-
Selective oestrogen receptor modulators (SERMs) for endometriosis.Cochrane Database Syst Rev. 2021 May 11;5(5):CD011169. doi: 10.1002/14651858.CD011169.pub2. Cochrane Database Syst Rev. 2021. PMID: 33973648 Free PMC article.
Cited by
-
Endometriosis: An Immunologist's Perspective.Int J Mol Sci. 2025 May 28;26(11):5193. doi: 10.3390/ijms26115193. Int J Mol Sci. 2025. PMID: 40508002 Free PMC article. Review.
References
-
- Skegro B, Bjedov S, Mikus M, et al. Endometriosis, pain and mental health. Psychiatry Danub. 2021;33(Suppl 4):632–636. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

