Discovery of anti-infective compounds against Mycobacterium marinum after biotransformation of simple natural stilbenes by a fungal secretome
- PMID: 39355425
- PMCID: PMC11443511
- DOI: 10.3389/fmicb.2024.1439814
Discovery of anti-infective compounds against Mycobacterium marinum after biotransformation of simple natural stilbenes by a fungal secretome
Abstract
Introduction: Mycobacterium tuberculosis (Mtb), the causative agent of tuberculosis, remains a serious threat to human health worldwide and the quest for new anti-tubercular drugs is an enduring and demanding journey. Natural products (NPs) have played a significant role in advancing drug therapy of infectious diseases.
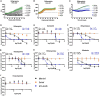
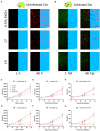
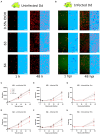
Methods: This study evaluated the suitability of a high-throughput infection system composed of the host amoeba Dictyostelium discoideum (Dd) and Mycobacterium marinum (Mm), a close relative of Mtb, to identify anti-infective compounds. Growth of Dd and intracellular Mm were quantified by using luminescence and fluorescence readouts in phenotypic assays. The system was first benchmarked with a set of therapeutic anti-Mtb antibiotics and then used to screen a library of biotransformed stilbenes.
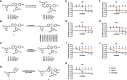
Results: The study confirmed both efficacy of established antibiotics such as rifampicin and bedaquiline, with activities below defined anti-mycobacterium susceptibility breakpoints, and the lack of activity of pyrazinamide against Mm. The screening revealed the promising anti-infective activities of trans-δ-viniferins and in particular of two compounds 17 and 19 with an IC50 of 18.1 μM, 9 μM, respectively. Both compounds had no activity on Mm in broth. Subsequent exploration via halogenation and structure-activity relationship studies led to the identification of derivatives with improved selectivity and potency. The modes of action of the anti-infective compounds may involve inhibition of mycobacterial virulence factors or boosting of host defense.
Discussion: The study highlights the potential of biotransformation and NP-inspired derivatization approaches for drug discovery and underscores the utility of the Dd-Mm infection system in identifying novel anti-infective compounds.
Keywords: Dictyostelium discoideum; Mycobacterium marinum; anti-infectives; natural products; phenotypic screening; stilbene derivatives.
Copyright © 2024 Nitschke, Huber, Vossio, Moreau, Marcourt, Gindro, Queiroz, Soldati and Hanna.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
LinkOut - more resources
Full Text Sources
Miscellaneous

