Nutrient Removal and Recovery from Urine Using Bio-Mineral Formation Processes
- PMID: 39355680
- PMCID: PMC11440639
- DOI: 10.1021/acssusresmgt.4c00025
Nutrient Removal and Recovery from Urine Using Bio-Mineral Formation Processes
Abstract
Harvesting nutrients from waste presents a promising initiative to advance and deliver the circular economy in the water sector while mitigating local shortages of mineral fertilizers worldwide. Urine, a small fraction of municipal wastewater, holds substantial amounts of nitrogen, orthophosphate (PO4-P), and chemical oxygen demand (COD). Separating urine aids targeted nutrient recovery, emissions reduction, and releasing capacity in wastewater treatment plants and taps into overlooked vital nutrients like magnesium (Mg2+) and potassium (K+), essential for plant growth. The ability of selected microorganisms (Brevibacterium antiquum, Bacillus pumilus, Halobacterium salinarum, Idiomarina loihiensis, and Myxococcus xanthus) to remove and recover nutrients from fresh urine through bio-mineral formation of struvite was investigated. The selected microorganisms outcompeted native microbes in open-culture fresh urine, and intact cell counts were 1.3 to 2.3 times larger than in noninoculated controls. PO4-P removal reached 50% after 4 days of incubation and 96% when urine was supplemented with Mg2+. Additionally, soluble COD was reduced by 60%; urea hydrolysis was only < 3% in controls, but it reached 35% in inoculated urine after 10 days. The dominant morphology of recovered precipitates was euhedral and prismatic, identified using energy dispersive spectroscopy and X-ray diffraction as struvite (i.e., bio-struvite), but K+ was also present at 5%. Up to 1 g bio-struvite/L urine was recovered. These results demonstrate the ability of bio-mineral producing microorganisms to successfully grow in urine and recover nutrients such as bio-struvite, that could potentially be used as sustainable fertilizers or chemicals.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

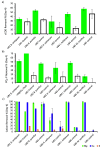


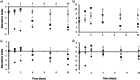


References
-
- Chipako T. L.; Randall D. G. Urine Treatment Technologies and the Importance of PH. Journal of Environmental Chemical Engineering 2020, 8 (1), 103622.10.1016/j.jece.2019.103622. - DOI
-
- Zang G. L.; Sheng G. P.; Li W. W.; Tong Z. H.; Zeng R. J.; Shi C.; Yu H. Q. Nutrient Removal and Energy Production in a Urine Treatment Process Using Magnesium Ammonium Phosphate Precipitation and a Microbial Fuel Cell Technique. Physical Chemistry Chemical Physics 2012, 14 (6), 1978–1984. 10.1039/c2cp23402e. - DOI - PubMed
-
- Sena M.; Hicks A. Life Cycle Assessment Review of Struvite Precipitation in Wastewater Treatment. Resources, Conservation and Recycling 2018, 139, 194–204. 10.1016/j.resconrec.2018.08.009. - DOI
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
