USP29 alleviates the progression of MASLD by stabilizing ACSL5 through K48 deubiquitination
- PMID: 39355870
- PMCID: PMC11791544
- DOI: 10.3350/cmh.2024.0478
USP29 alleviates the progression of MASLD by stabilizing ACSL5 through K48 deubiquitination
Abstract
Background/aims: Metabolic dysfunction-associated steatotic liver disease (MASLD) is a chronic liver disease characterized by hepatic steatosis. Ubiquitin-specific protease 29 (USP29) plays pivotal roles in hepatic ischemiareperfusion injury and hepatocellular carcinoma, but its role in MASLD remains unexplored. Therefore, the aim of this study was to reveal the effects and underlying mechanisms of USP29 in MASLD progression.
Methods: USP29 expression was assessed in liver samples from MASLD patients and mice. The role and molecular mechanism of USP29 in MASLD were assessed in high-fat diet-fed and high-fat/high-cholesterol diet-fed mice and palmitic acid and oleic acid treated hepatocytes.
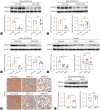
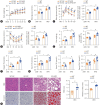
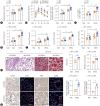
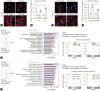
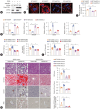
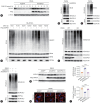
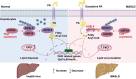
Results: USP29 protein levels were significantly reduced in mice and humans with MASLD. Hepatic steatosis, inflammation and fibrosis were significantly exacerbated by USP29 deletion and relieved by USP29 overexpression. Mechanistically, USP29 significantly activated the expression of genes related to fatty acid β-oxidation (FAO) under metabolic stimulation, directly interacted with long-chain acyl-CoA synthase 5 (ACSL5) and repressed ACSL5 degradation by increasing ACSL5 K48-linked deubiquitination. Moreover, the effect of USP29 on hepatocyte lipid accumulation and MASLD was dependent on ACSL5.
Conclusion: USP29 functions as a novel negative regulator of MASLD by stabilizing ACSL5 to promote FAO. The activation of the USP29-ACSL5 axis may represent a potential therapeutic strategy for MASLD.
Keywords: ACSL5 protein, human; Lipid metabolism disorders; Metabolic dysfunction-associtaed steatotic liver disease; USP29 protein, human.
Conflict of interest statement
The authors have no conflicts to disclose.
Figures


References
-
- Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M, et ak. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15:11–20. - PubMed
-
- Powell EE, Wong VW, Rinella M. Non-alcoholic fatty liver disease. Lancet. 2021;397:2212–2224. - PubMed
-
- European Association for the Study of the Liver. European Association for the Study of Diabetes. European Association for the Study of Obesity EASL-EASD-EASO clinical practice guidelines on the management of metabolic dysfunction-associated steatotic liver disease (MASLD): executive summary. Diabetologia. 2024;67:2375–2392. - PMC - PubMed
-
- Riazi K, Azhari H, Charette JH, Underwood FE, King JA, Afshar EE, et al. The prevalence and incidence of NAFLD worldwide: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2022;7:851–861. - PubMed
-
- Yong JN, Lim WH, Ng CH, Tan DJH, Xiao J, Tay PWL, et al. Outcomes of nonalcoholic steatohepatitis after liver transplantation: an updated meta-analysis and systematic review. Clin Gastroenterol Hepatol. 2023;21:45–54.e6. - PubMed
MeSH terms
Substances
Grants and funding
- 81970011/National Science Foundation of China
- 82170595/National Science Foundation of China
- ZNLH202211/Basic Medicine-Clinical Medicine Transformation Collaborative Fund of Zhongnan Hospital of Wuhan University
- ZNLH202204/Basic Medicine-Clinical Medicine Transformation Collaborative Fund of Zhongnan Hospital of Wuhan University
- GDXZ2020006/Henan Charity Federation Hepatobiliary Fund
LinkOut - more resources
Full Text Sources
Medical
Research Materials

