Pharmacokinetic, Safety, and Pharmacodynamic Profiles of Saroglitazar Magnesium in Cholestatic Cirrhosis With Hepatic Impairment and Participants With Renal Impairment
- PMID: 39355940
- PMCID: PMC11652809
- DOI: 10.1002/cpt.3450
Pharmacokinetic, Safety, and Pharmacodynamic Profiles of Saroglitazar Magnesium in Cholestatic Cirrhosis With Hepatic Impairment and Participants With Renal Impairment
Abstract
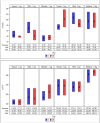
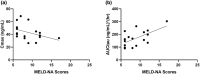
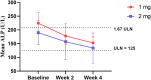
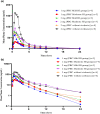
Saroglitazar magnesium, a dual PPAR α/γ agonist, currently in Phase III for treating primary biliary cholangitis (PBC), was evaluated for its pharmacokinetic (PK) profile, safety, and pharmacodynamics in participants with cholestatic liver disease (CLD) across different levels of hepatic impairment (HI) and participants with severe renal impairment (RI). Three PK studies comparing saroglitazar with healthy controls were conducted: Study 1 involved daily oral doses of 1 or 2 mg for 4 weeks in 12 PBC cirrhosis participants with mild or moderate HI; Study 2 assessed single-dose PK (2 or 4 mg) in eight non-cirrhotic CLD participants; Study 3 evaluated single-dose PK (2 mg) in eight participants with severe RI. On day 1, saroglitazar exposure increased by 14.6-42% in mild HI vs. normal, but by day 28, levels were similar, indicating no accumulation. In moderate HI, exposure was significantly increased by 50.4-85% on days 1 and 28, with 34-46% lower clearance despite a similar half-life. The moderate HI group had a 59% higher exposure than the non-cirrhotic group. Saroglitazar (1 and 2 mg) reduced alkaline phosphatase (ALP) levels by 17-40% after 4 weeks in participants with abnormal baseline ALP. Single-dose PK in non-cirrhotic CLD (2 and 4 mg) and severe RI (2 mg) was comparable to matched controls without significant safety issues. Overall, saroglitazar (1 and 2 mg) was safe and well-tolerated in cholestatic cirrhosis with mild HI and participants with severe RI without major PK changes. Moderate HI increased exposure and decreased clearance without any safety concerns.
© 2024 The Author(s). Clinical Pharmacology & Therapeutics published by Wiley Periodicals LLC on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
Raj Vuppalanchi receives institutional funding from Zydus Therapeutics, Eli Lilly, Terns, AstraZeneca, Takeda, Gilead Sciences, and Galectin Therapeutics. Additionally, he provides consultation services to Fortrea, Medpace, Cour Pharmaceuticals, Regeneron, Avante Sante, and GSK, where he serves as a member of hepatic adjudication and data safety monitoring committees. Mary M. Cruz has no conflict of interest. Taufik Momin and Harilal Patel are employees of Zydus Lifesciences Ltd., whereas Farheen Shaikh, Kimberly Swint, and Deven Parmar are employees of Zydus Therapeutics Inc.
Figures
References
-
- Lindor, K.D. , Bowlus, C.L. , Boyer, J. , Levy, C. & Mayo, M. Primary biliary cholangitis: 2021 practice guidance update from the American Association for the Study of Liver Diseases. Hepatology 75, 1012–1013 (2021). - PubMed
-
- Alvaro, D. , Carpino, G. , Craxi, A. , Floreani, A. , Moschetta, A. & Invernizzi, P. Primary biliary cholangitis management: controversies, perspectives, and daily practice implications from an expert panel. Liver Int. 40, 2590–2601 (2020). - PubMed
-
- European Association for the Study of the Liver . EASL Clinical Practice Guidelines: the diagnosis and management of patients with primary biliary cholangitis. J. Hepatol. 67, 145–172 (2017). - PubMed
-
- Lu, M. et al. Increasing prevalence of primary biliary cholangitis and reduced mortality with treatment. Clin. Gastroenterol. Hepatol. 16, 1342–1350.e1 (2018). - PubMed
-
- Chen, R. , Tang, R. , Ma, X. & Gershwin, M.E. Immunologic responses and the pathophysiology of primary biliary cholangitis. Clin. Liver Dis. 26, 583–611 (2022). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

