Targeting the Epidermal Growth Factor Receptor Pathway in Chemotherapy-Resistant Triple-Negative Breast Cancer: A Phase II Study
- PMID: 39356138
- PMCID: PMC11520071
- DOI: 10.1158/2767-9764.CRC-24-0255
Targeting the Epidermal Growth Factor Receptor Pathway in Chemotherapy-Resistant Triple-Negative Breast Cancer: A Phase II Study
Abstract
Purpose: Epidermal growth factor receptor (EGFR) pathway activation causes chemotherapy resistance, and inhibition of the EGFR pathway sensitizes triple-negative breast cancer (TNBC) cells to chemotherapy in preclinical models. Given the high prevalence of EGFR overexpression in TNBC, we conducted a single-arm phase II study of panitumumab (anti-EGFR monoclonal antibody), carboplatin, and paclitaxel as the second phase of neoadjuvant therapy (NAT) in patients with doxorubicin and cyclophosphamide (AC)-resistant TNBC (NCT02593175).
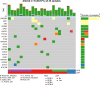
Patients and methods: Patients with early-stage, AC-resistant TNBC, defined as disease progression or ≤80% reduction in tumor volume after four cycles of AC, were eligible for this study and received panitumumab (2.5 mg/kg i.v., every week × 13), paclitaxel (80 mg/m2 i.v. every week × 12), and carboplatin (AUC = 4 i.v., every 3 weeks × 4) as the second phase of NAT. A two-stage Gehan-type design was used to detect an improvement in the pathological complete response (pCR)/residual cancer burden class I (RCB-I) rate from 5% to 20%. Whole-exome sequencing was performed on diagnostic tumor biospecimens, where available.
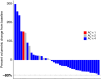
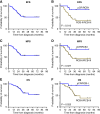
Results: From November 3, 2016, through August 23, 2021, 43 patients with AC-resistant TNBC were enrolled. The combined pCR/RCB-I rate was 30.2%. The most common treatment-related adverse events were neutropenia (72%) and anemia (61%), with 7 (16%), 16 (37%), and 8 (19%) patients experiencing grade 4 neutropenia, grade 3 neutropenia, and grade 3 anemia, respectively. No new safety signals were observed.
Conclusions: This study met its primary endpoint (pCR/RCB-I = 30.2% vs. 5% in historical controls), suggesting that panitumumab should be evaluated as a component of NAT in patients with chemotherapy-resistant TNBC in a larger, randomized clinical trial.
Significance: The epidermal growth factor receptor (EGFR) pathway has been implicated as a driver of chemotherapy resistance in triple-negative breast cancer (TNBC). Here, we evaluate the combination of panitumumab, carboplatin, and paclitaxel as the second phase of neoadjuvant therapy (NAT) in patients with AC-resistant TNBC. This study met its primary efficacy endpoint, and molecular alterations in EGFR pathway genes did not seem to influence response to the study regimen.
©2024 The Authors; Published by the American Association for Cancer Research.
Conflict of interest statement
C. Yam reports grants from Amgen during the conduct of the study, as well as grants from Genentech, BostonGene, Sanofi, Pfizer, Astellas, and Novartis, grants and other support from Gilead, and personal fees from MDHealth Brazil and Binaytara Foundation outside the submitted work. E. Kong reports grants from National Library of Medicine during the conduct of the study. S. Damodaran reports grants from Sermonix, EMD Serono, AstraZeneca, Taiho Oncology, Daiichi Sankyo, Medilink Therapeutics, Duality Biologics, and Novartis outside the submitted work. R.P. Candelaria reports that funding sources to the institution for the submitted work have been declared on the title page of the article by the corresponding author. He did not personally receive additional payments or services from a third party. E.A. Mittendorf reports other support from AstraZeneca, BioNTech, Merck, Moderna, Bristol Myers Squibb, Roche/Genentech, and Merck Sharp & Dohme, nonfinancial support from Gilead, and grants from Roche/Genentech outside the submitted work. A.M. Thompson reports other support from Eli Lilly and Company outside the submitted work. E.E. Ravenberg reports other from Eli Lilly and Company outside the submitted work, as well as employment with Eli Lilly and Company and receiving compensation and stock options. J.K. Litton reports grants from Pfizer, Merck, Genentech, AstraZeneca, and Zenith outside the submitted work. V. Valero reports grants and other support from MD Anderson Cancer Center during the conduct of the study. D. Tripathy reports grants and personal fees from Novartis and Ambryx and personal fees from Pfizer, AstraZeneca, Stemline-Menarini, Personalis, Sermonix, Roche, Gilead, Puma Biotechnology, BeiGene, Jazz Pharmaceuticals, and Zetagen outside the submitted work. A. Korkut reports grants from BostonGene outside the submitted work. S.L. Moulder reports other support from Eli Lilly and Company during the conduct of the study, as well as other support from Eli Lilly and Company outside the submitted work. L. Huo reports other support from MD Anderson Cancer Center during the conduct of the study. B. Lim reports serving in a consultancy/advisory role for Celcuity, Natera, Daiichi Sankyo, Novartis, Pfizer, and AstraZeneca; honoraria from Puma Biotechnology, Novartis, and Pfizer; and grant/research funding from Genentech, Takeda, Merck, Celcuity, Eli Lilly and Company, Puma Biotechnology, and Calithera Biosciences. N.T. Ueno reports other support from Pear Bio and Phoenix Molecular Designs, personal fees from Kyowa Hakko Kirin, Pfizer, Bayer, MEDSIR, Genomic Health, Bristol Myers Squibb, AstraZeneca, Carna Biosciences, Eisai, Bayer, Gilead, Eli Lilly and Company, and Oncocyte, grants from Amgen and Preferred Medicine, and grants and personal fees from Daiichi Sankyo outside the submitted work. No disclosures were reported by the other authors.
Figures
References
-
- Schmid P, Cortes J, Pusztai L, McArthur H, Kümmel S, Bergh J, et al. Pembrolizumab for early triple-negative breast cancer. N Engl J Med 2020;382:810–21. - PubMed
-
- Schmid P, Cortes J, Dent R, Pusztai L, McArthur H, Kümmel S, et al. Event-free survival with pembrolizumab in early triple-negative breast cancer. N Engl J Med 2022;386:556–67. - PubMed
-
- Pusztai L, Denkert C, O’Shaughnessy J, Cortes J, Dent R, McArthur H, et al. Event-free survival by residual cancer burden with pembrolizumab in early-stage TNBC: exploratory analysis from KEYNOTE-522. Ann Oncol 2024;35:429–36. - PubMed
-
- Chan TO, Rittenhouse SE, Tsichlis PN. AKT/PKB and other D3 phosphoinositide-regulated kinases: kinase activation by phosphoinositide-dependent phosphorylation. Annu Rev Biochem 1999;68:965–1014. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

