Amiodarone or Implantable Cardioverter-Defibrillator in Chagas Cardiomyopathy: The CHAGASICS Randomized Clinical Trial
- PMID: 39356542
- PMCID: PMC11447631
- DOI: 10.1001/jamacardio.2024.3169
Amiodarone or Implantable Cardioverter-Defibrillator in Chagas Cardiomyopathy: The CHAGASICS Randomized Clinical Trial
Abstract
Importance: Over 10 000 people with Chagas disease experience sudden cardiac death (SCD) annually, mostly caused by ventricular fibrillation. Amiodarone hydrochloride and the implantable cardioverter-defibrillator (ICD) have been empirically used to prevent SCD in patients with chronic Chagas cardiomyopathy.
Objective: To test the hypothesis that ICD is more effective than amiodarone therapy for primary prevention of all-cause mortality in patients with chronic Chagas cardiomyopathy and moderate to high mortality risk, assessed by the Rassi score.
Design, setting, and participants: CHAGASICS is an open-label, randomized clinical trial. The study enrolled patients from 13 centers in Brazil from May 30, 2014, to August 13, 2021, with the last follow-up November 8, 2021. Patients with serological findings positive for Chagas disease, a Rassi risk score of at least 10 points (intermediate to high risk), and at least 1 episode of nonsustained ventricular tachycardia were eligible to participate. Data were analyzed from May 3, 2022, to June 16, 2023.
Interventions: Patients were randomized 1:1 to receive ICD or amiodarone (with a loading dose of 600 mg after randomization).
Main outcomes and measures: The primary outcome was all-cause mortality, and secondary outcomes included SCD, hospitalization for heart failure, and necessity of a pacemaker during the entire follow-up.
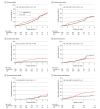
Results: The study was stopped prematurely for administrative reasons, with 323 patients randomized (166 in the amiodarone group and 157 in the ICD group), rather than the intended 1100 patients. Analysis was by intention to treat at a median follow-up of 3.6 (IQR, 1.8-4.4) years. Mean (SD) age was 57.4 (9.8) years, 185 patients (57.3%) were male, and the mean (SD) left ventricular ejection fraction was 37.0% (11.6%). There were 60 deaths (38.2%) in the ICD arm and 64 (38.6%) in the amiodarone group (hazard ratio [HR], 0.86 [95% CI, 0.60-1.22]; P = .40). The rates of SCD (6 [3.8%] vs 23 [13.9%]; HR, 0.25 [95% CI, 0.10-0.61]; P = .001), bradycardia requiring pacing (3 [1.9%] vs 27 [16.3%]; HR, 0.10 [95% CI, 0.03-0.34]; P < .001), and heart failure hospitalization (14 [8.9%] vs 28 [16.9%]; HR, 0.46 [95% CI, 0.24-0.87]; P = .01) were lower in the ICD group compared with the amiodarone arm.
Conclusions and relevance: In patients with chronic Chagas cardiomyopathy at moderate to high risk of mortality, ICD did not reduce the risk of all-cause mortality. However, ICD significantly reduced the risk of SCD, pacing need, and heart failure hospitalization compared with amiodarone therapy. Further studies are warranted to confirm the evidence generated by this trial.
Trial registration: ClinicalTrials.gov Identifier: NCT01722942.
Conflict of interest statement
Figures


References
-
- World Health Organization. World Chagas Disease Day 2023. to focus on integrating universal care and surveillance at the primary care level. Accessed November 17, 2023. https://www.who.int/news/item/14-04-2023-world-chagas-disease-day-2023-t....
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

