Ultrafast transient absorption spectra and kinetics of human blue cone visual pigment at room temperature
- PMID: 39356673
- PMCID: PMC11474067
- DOI: 10.1073/pnas.2414037121
Ultrafast transient absorption spectra and kinetics of human blue cone visual pigment at room temperature
Abstract
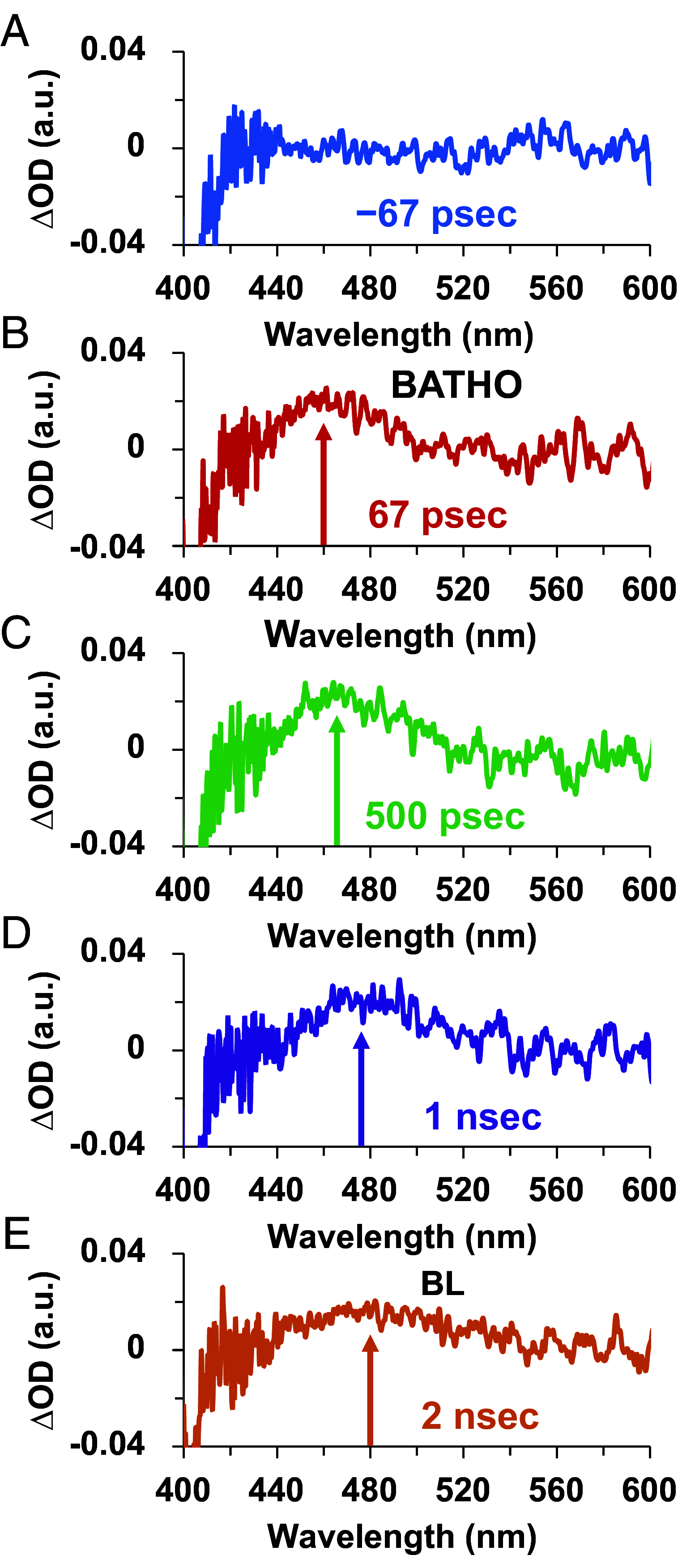
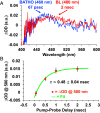
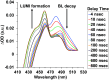
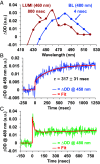
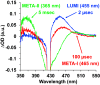
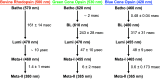
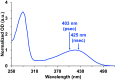
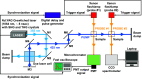
The ultrafast photochemical reaction mechanism, transient spectra, and transition kinetics of the human blue cone visual pigment have been recorded at room temperature. Ultrafast time-resolved absorption spectroscopy revealed the progressive formation and decay of several metastable photo-intermediates, corresponding to the Batho to Meta-II photo-intermediates previously observed with bovine rhodopsin and human green cone opsin, on the picosecond to millisecond timescales following pulsed excitation. The experimental data reveal several interesting similarities and differences between the photobleaching sequences of bovine rhodopsin, human green cone opsin, and human blue cone opsin. While Meta-II formation kinetics are comparable between bovine rhodopsin and blue cone opsin, the transition kinetics of earlier photo-intermediates and qualitative characteristics of the Meta-I to Meta-II transition are more similar for blue cone opsin and green cone opsin. Additionally, the blue cone photo-intermediate spectra exhibit a high degree of overlap with uniquely small spectral shifts. The observed variation in Meta-II formation kinetics between rod and cone visual pigments is explained based on key structural differences.
Keywords: blue cone opsin; cone photo-intermediates; cone visual pigments; phototransduction; ultrafast spectroscopy.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures

References
-
- Arshavsky V. Y., Like night and day: Rods and cones have different pigment regeneration pathways. Neuron 36, 1–3 (2002). - PubMed
-
- Kawamura S., Tachibanaki S., Molecular bases of rod and cone differences. Prog. Retin. Eye Res. 90, 101040 (2022). - PubMed
-
- Ebrey T., Koutalos Y., Vertebrate photoreceptors. Prog. Retin. Eye Res. 20, 49–94 (2001). - PubMed

