Neuroinflammation as a cause of differential Müller cell regenerative responses to retinal injury
- PMID: 39356769
- PMCID: PMC11446274
- DOI: 10.1126/sciadv.adp7916
Neuroinflammation as a cause of differential Müller cell regenerative responses to retinal injury
Abstract
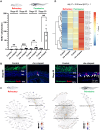
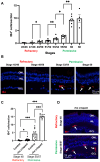
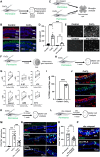
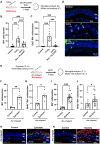
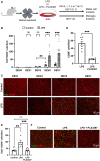
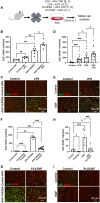
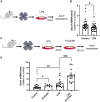
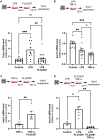
Unlike mammals, some nonmammalian species recruit Müller glia for retinal regeneration after injury. Identifying the underlying mechanisms may help to foresee regenerative medicine strategies. Using a Xenopus model of retinitis pigmentosa, we found that Müller cells actively proliferate upon photoreceptor degeneration in old tadpoles but not in younger ones. Differences in the inflammatory microenvironment emerged as an explanation for such stage dependency. Functional analyses revealed that enhancing neuroinflammation is sufficient to trigger Müller cell proliferation, not only in young tadpoles but also in mice. In addition, we showed that microglia are absolutely required for the response of mouse Müller cells to mitogenic factors while negatively affecting their neurogenic potential. However, both cell cycle reentry and neurogenic gene expression are allowed when applying sequential pro- and anti-inflammatory treatments. This reveals that inflammation benefits Müller glia proliferation in both regenerative and nonregenerative vertebrates and highlights the importance of sequential inflammatory modulation to create a regenerative permissive microenvironment.
Figures

References
-
- Bringmann A., Pannicke T., Grosche J., Francke M., Wiedemann P., Skatchkov S. N., Osborne N. N., Reichenbach A., Müller cells in the healthy and diseased retina. Prog. Retin. Eye Res. 25, 397–424 (2006). - PubMed
-
- Langhe R., Chesneau A., Colozza G., Hidalgo M., Ail D., Locker M., Perron M., Müller glial cell reactivation in Xenopus models of retinal degeneration. Glia 65, 1333–1349 (2017). - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources

